FlexX is shorthand for “Flexible Beamline for Macromolecular Crystallography,” where the second, capital “X” stems from a common shorthand for “Crystal,” or “X-tal.” The name also indicates the flexibility of the beamline in accommodating a wide variety of crystallography-related experiments. Finke and the beamline work to accommodate challenging experiments that would be difficult to perform at other beamlines across the U.S.
Research at FlexX is centered around X-ray crystallography, particularly protein and nucleic acid crystallography, though the beamline can perform a wide variety of crystallographic experiments.
FlexX researchers are primarily interested in structural and molecular biology - looking at the atomic structure of proteins, nucleic acids, and complexes that contain the two. The better that researchers understand the atomic structure of proteins and nucleic acids, the more they understand how they interact with other proteins and with small molecules, such as drug molecules, and their function at specific biological sites.
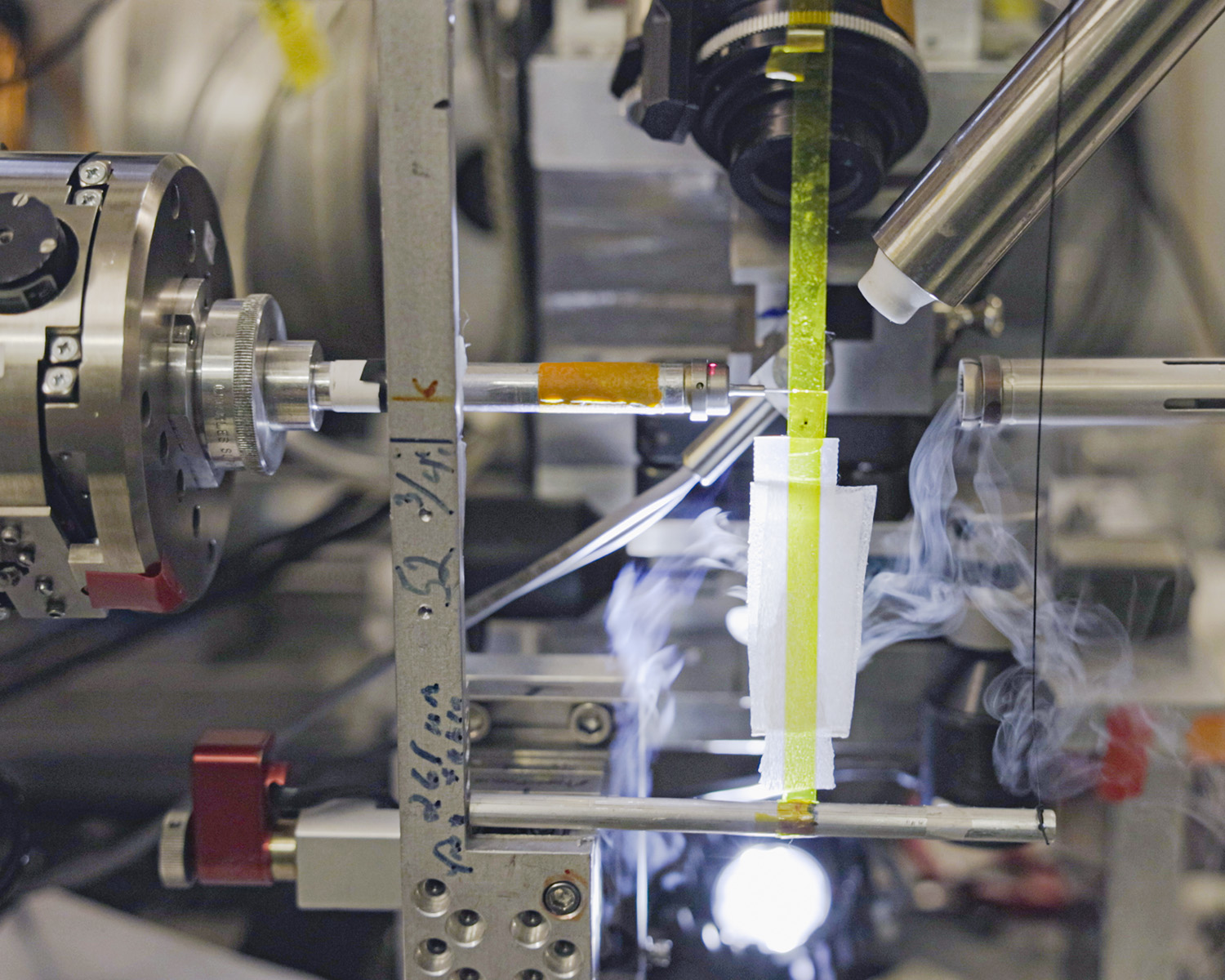
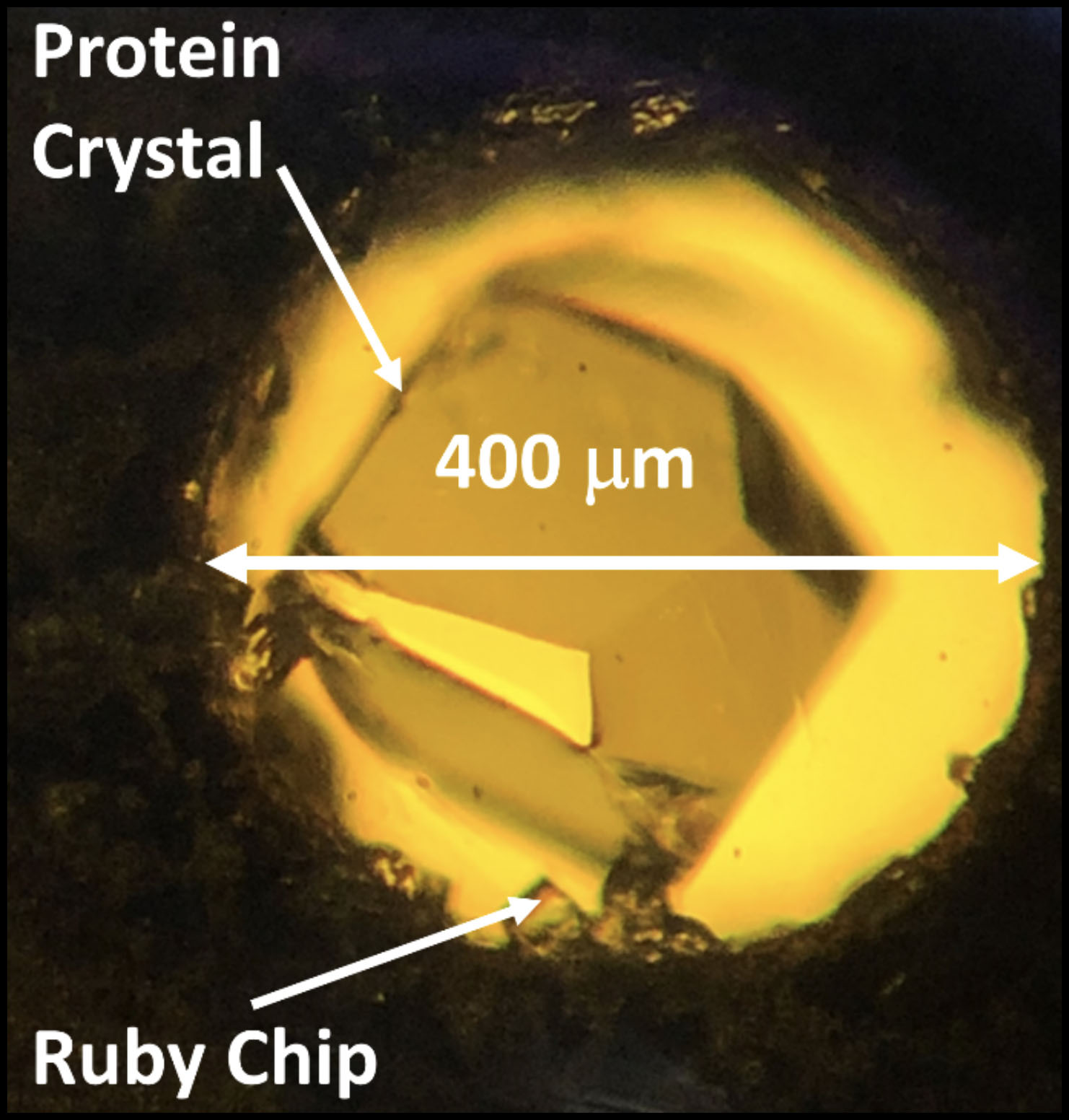
Before performing their experiments at FlexX, researchers grow crystals of the protein or biological macromolecule that they are interested in studying. Those crystals are then brought to FlexX and held in place on a goniometer and rotated while being exposed to X-rays. The X-rays are diffracted by the crystal and the diffraction pattern is recorded on a large-area X-ray detector. The atomic structure of the macromolecules that make up the crystal can then be back-calculated based on the diffraction pattern that was recorded.
The majority of experiments conducted at FlexX fall into three categories: standard crystallography, room temperature crystallography, and high pressure crystallography. Standard crystallography (or standard cryocrystallography) experiments take place at 100 Kelvin using protein crystals that have been cryo-cooled in liquid nitrogen. The majority of protein crystallography experiments fall into this category.
Room temperature crystallography requires some finesse compared to cryocrystallography, because the intense X-ray beams produced at light sources like CHESS deteriorate and damage protein crystals (and, thus, their diffraction patterns) quickly at room temperature. The damage is slowed considerably at lower temperatures. A protein crystal might be able to diffract while being irradiated by X-rays for several minutes at 100 Kelvin, but would lose its ability to diffract X-rays with only seconds of irradiation at room temperature. However, crystallography at room temperature opens the door to many important insights, such as unique modes of binding of drugs, protein dynamics (how proteins fold and interact as a function of temperature), and kinetics (studying the speed at which biochemical processes occur). In order to get around this hurdle, room temperature crystallography utilizes techniques such as single crystal crystallography with high scan rates and, especially, serial crystallography, which is a technique where small diffraction data sets from a large number of protein crystals are merged together.
The third type of experiment, high pressure crystallography, uses a diamond anvil cell to produce very high pressures on protein crystals. This technique simulates pressures that would be found, for example, at the bottom of the ocean, where the majority of the world’s biomass exists. These life forms have adapted to thrive at such high pressures, to the point where they would simply not survive at atmospheric pressure. In fact, many biologists think that complex life on Earth got its start at the bottom of the ocean! Understanding how and why life thrives at high pressure is the primary scientific goal of this technique.
Although all the research conducted at FlexX has broad-reaching implications, perhaps none is more relevant to most people than that involving drug development. FlexX research leads to a better understanding of the three-dimensional structure of enzymes, drug complexes, and drug interactions, leading to better drug development to promote or inhibit specific enzymatic pathways.
A specific example of FlexX’s impact on drug research took place near the beginning of the Covid-19 pandemic, when the beamline was given special permission to resume operations in May 2020 in order to study a class of drugs that are potential Sars-CoV-2 antivirals. At that time, cancer researcher Rick Cerione had been studying drugs that target the same enzymatic pathway used by Covid-19. There were two drugs that showed vastly different efficacy in clinical cancer trials, but looked almost identical under standard crystallography experiments. In collaboration with Cerione, Finke developed room temperature crystallography methods to see if they could parse out a difference between the drugs in conditions closer to the real world. “We were able to determine a difference, but we only ever saw it at room temperature,” says Finke.
These types of crystallographic experiments are usually done at around 100 K because X-rays damage proteins and protein crystals, so that diffraction is lost very quickly at room temperature. However, Finke has developed methods for conducting serial crystallography at room temperature by using a large number of protein crystals in succession and gleaning a small amount of data from each crystal, then combining the data to generate a “radiation damage-free” structure. As Finke explains, “it’s a way to circumvent this radiation damage problem that’s inherent in protein crystallography.” This research has enabled Cerione and his team to better understand their target drugs’ interactions with proteins and their potential for treatment.
Most CHESS beamlines made changes at the start of the Covid-19 pandemic in order to accommodate remote research, but MacCHESS and FlexX users have been collecting data since well before the pandemic. The MacCHESS beamlines were the first CHESS beamlines to enable routine, remote beamline operation for users nearly a decade ago. The option has been very popular with researchers - approximately half of the FlexX user base collected data remotely before the pandemic. MacCHESS makes it as simple as possible for users by giving them complete control of the data collection desktop when logged in. Users send in their samples, frozen in liquid nitrogen, and then a robot automounter picks up each individual sample and loads it for analysis, meaning data collection requires no human intervention at the beamline and users can collect their data fully remotely.

FlexX’s remote data collection has not changed much since before the pandemic, but Finke and CHESS operations staff have added more and better streamlined communication tools, allowing users to communicate with CHESS operators and scientists 24 hours a day.
FlexX is currently in the process of implementing new software and data collection graphical user interfaces that will allow for streamlined data collection and other exciting new features for users. The planned upgrades will also give users the ability to raster across large crystals and find the best spot for data collection on a single crystal. Also in the pipeline at FlexX are new technologies and experimental setups for room temperature crystallography and high pressure crystallography, improving the beamline’s capabilities in both areas.
Finke’s favorite aspect of working with FlexX researchers is that his position gives him a “bird's eye view of the different research projects that people are working on and the challenges that are associated with the latest and greatest research in structural biology… It’s very exciting to see not only where structural biology is headed in the next few years, but also how I can help assist with new experiments, new technologies, and new ideas.”
