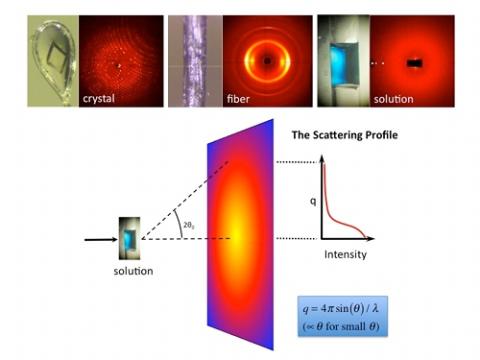
Most X-rays travel right through matter without changing direction, but approximately one out of every million gets deflected at a small angle! The very faint “small angle" scattering pattern on an X-ray detector resembles a sunset, with the undeflected beam being like the sun; there is a bright glow nearest the beam that fades to dark at higher angles. To protect our sensitive detector, we block the direct beam with a small metal beamstop. With modern algorithms, the simple pattern of light to dark on the X-ray detector can yield a surprising amount of valuable structural information about biomolecules. In fact, BioSAXS is becoming an indispensable tool in biomedical science. Not only can researchers determine the mass and size of a biomolecule in solution, but they can reconstruct its basic shape, tell if it is rigid or flexible, and even figure out how multiple molecules fit together to form complex molecular machines. Increasingly, advances in medical research depend upon gaining a clear understanding of how biomolecules function and interact within the living cell. BioSAXS is one of the few techniques that can yield structural information on how biomolecules behave under native conditions akin to that of the living cell.
What is BioSAXS?
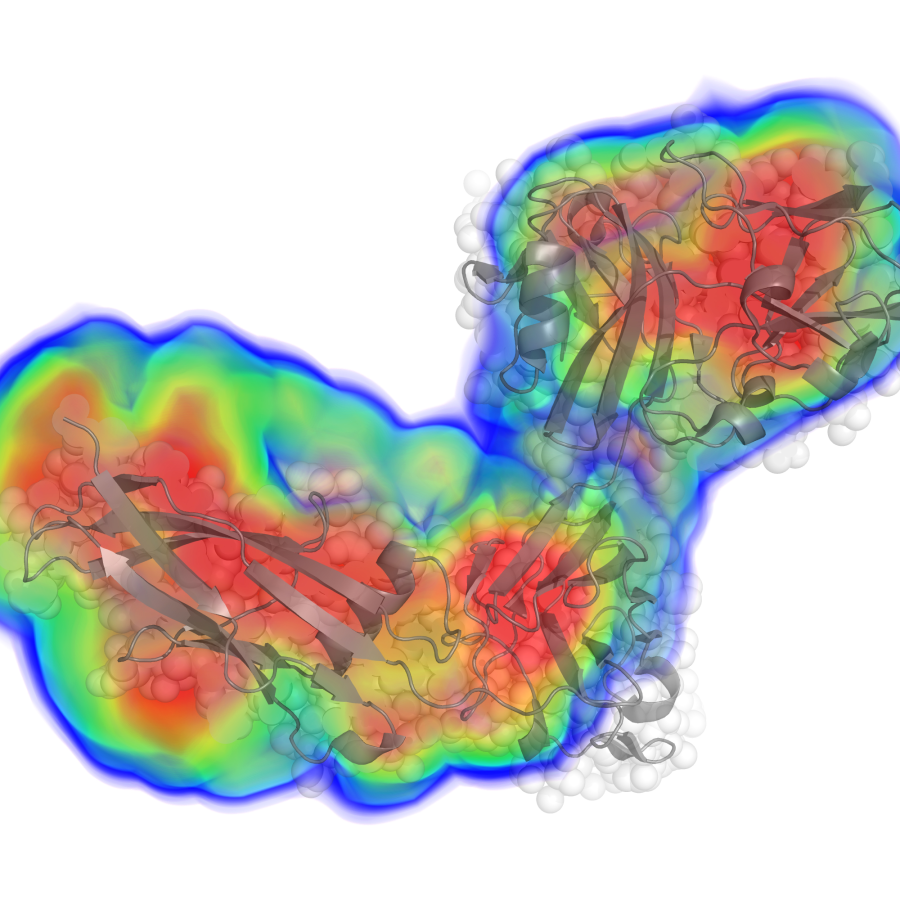
What parameters can BioSAXS determine?
- radius of gyration (typically 2% accuracy)
- molecular weight (typically 10% accuracy)
- maximum intra-particle distance
- low-resolution electron density
- degree of folding, denaturation, or disorder
- comparisons with models
- interparticle interaction potentials
What are people able to do with BioSAXS data?
- determine physiological oligomeric state
- validate proposed models of complexes
- build complexes from monomers or known fragments
- study protein-protein interaction under different solution conditions
- model missing loops and domains
- refine homology models
- categorize discrete folded and unfolded states
- find volume fractions in mixtures
Will BioSAXS work on my samples?
In crystallography, poor crystals and overlapping spots are a frequent cause of failure. BioSAXS can have problems too. Just because sample preparation is less extensive does not necessarily ensure success. In crystallography, it is easier to detect bad data: you can't index or integrate the diffraction spots. With BioSAXS, however, you can process bad data with very few indications that anything is wrong. It is therefore very important to understand how to recognize bad data and to diagnose the possible causes.