Beyond the usual

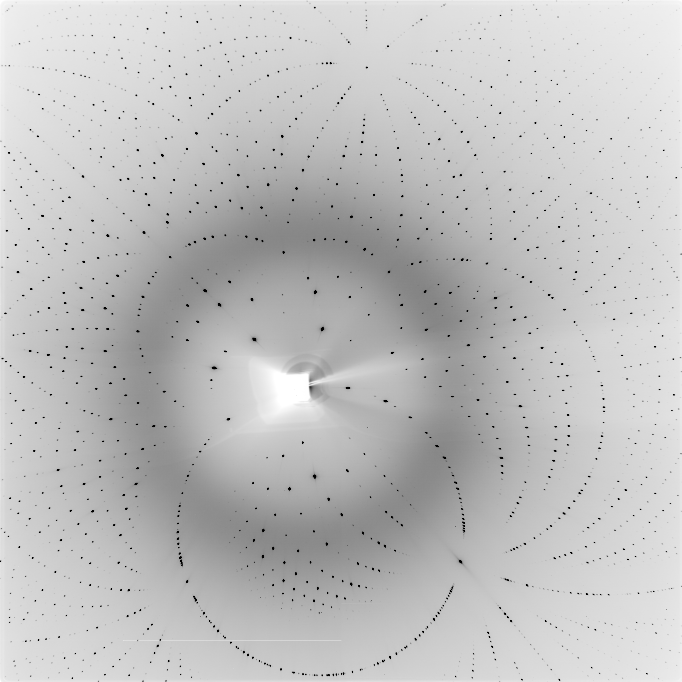
Experiments under pressure in diamond anvil cell (DAC). Test image taken using a lysozyme crystal; pressure 400 MPA, 5-second exposure, 1° oscillation.
Serial Crystallography
Serial crystallography at MacCHESS
MacCHESS offers users an advanced serial microcrystallography experience.
Diffuse scattering experiments
Enzyme Dynamics beyond Bragg Diffraction
Nozomi Ando, Ph.D., Assistant Professor of Chemistry & Chemical Biology,
Cornell University, Department of Chemistry & Chemical Biology
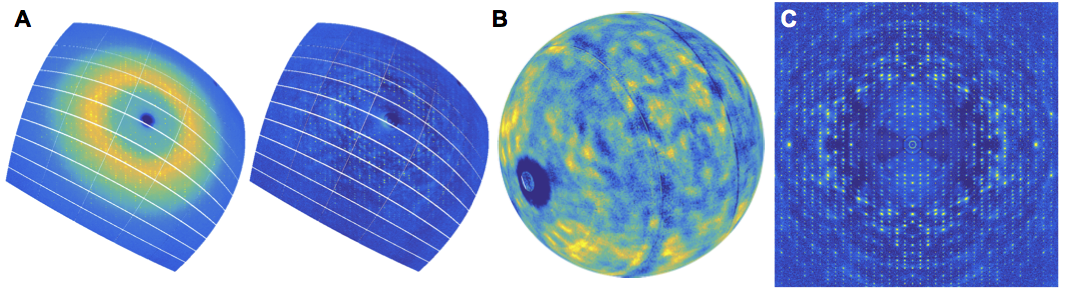
X-ray crystallography has proven to be an invaluable tool in structural and mechanistic studies of enzymes. However, our knowledge of how protein dynamics affect function is largely incomplete. Although Bragg diffraction can indicate mobility in a protein structure, it does not contain information on how motions occur in concert. To access this information, we must venture beyond Bragg diffraction into a complex, relatively unexplored region of X-ray data known as diffuse scattering. All proteins are dynamic, even inside crystals. Non-periodic patterns in the lattice arising from correlated motions leads to a cloudy, textured background pattern that is routinely thrown away in conventional crystallography.
References:
1. Das, K., and Arnold, E. (2013) HIV-1 reverse transcriptase and antiviral drug resistance. Part 1. Curr. Opin. Virol. 3, 111-118.
2. Sarafianos, S.G., Marchand, B., Das, K., Himmel, D., Parniak, M.A., Hughes, S.H., and Arnold E. (2009) Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J. Mo.l Biol. 385, 693-713.
3. Temiz, N.A., and Bahar, I. (2002) Inhibitor binding alters the directions of domain motions in HIV-1 reverse transcriptase. Proteins 49, 61-70.
4. Shen, L., Shen, J., Luo, X., Cheng, F., Xu, Y., Chen, K., Arnold, E., Ding, J., and Jiang, H. (2003) Steered molecular dynamics simulation on the binding of NNRTI to HIV-1 RT. Biophys. J. 84, 3547-3563.
5. Kung, Y., Ando, N., Doukov, T.I., Blasiak, L.C., Bender, G., Seravalli, J., Ragsdale, S.W., and Drennan, C.L. (2012) Visualizing molecular juggling within a B12-dependent methyltransferase complex. Nature 484, 265-269. PMCID: PMC3326194
6. Zhang, Y., and Stubbe, J. (2011) Bacillus subtilis class 1b Ribonucleotide Reductase is a dimangansese(III)-tyrosyl radical enzyme. Biochemistry 50, 5615-5623.
7. Jarrett, J.T., Huang, S., and Matthews, R.G. (1998) Methionine synthase exists in two distinct conformations that differ in reactivity towards methyletrahydrofolate, adenosylmethionine and flavodoxin. Bichemistry 37, 5372-5382.
8. Meisburger S.P., Ando N., "Correlated Motions from Crystallography beyond Diffraction," Accounts of Chemical Research 50(3), 580-583, 2017.
Use of SAXS to study the hydration of gels.
Observing molecular alignment in biofilms
Biofilms are communities of microorganisms that stick to surfaces using films of natural polymers such as polysaccharides. How microorganisms navigate and organize within the polymer is a subject of great importance in both in medicine and industry. X-ray scattering is a sensitive probe of alignment of molecules and the formation of order in soft materials. Using a novel inclined ring support, controlled compression of polysaccharide gels can reveal induced alignment of fibers that has important implications for how bacteria spread within the gel. MacCHESS is experienced with creating novel sample support and manipulation setups integrated with high-sensitivity X-ray scattering detection and strong data processing support software.
References:
David J. Lemon, Xingbo Yang, Pragya Srivastava, Yan-Yeung Luk & Anthony G. Garza. Polymertropism of rod-shaped bacteria: movement along aligned polysaccharide fibers. Scientific Reports 7, Article number: 7643 (2017) doi:10.1038/s41598-017-07486-0.
Users interested in studying gels and other biologically-related soft matter should contact Richard Gillilan to discuss feasibility.
Irradiation of virus samples with X-rays, as a test of alternative means of inactivating the virus.

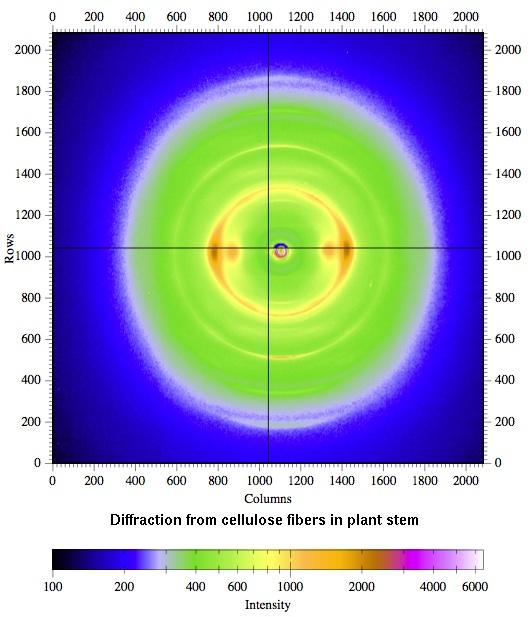
Diffraction from cellulose fibers in plant stems, to determine the crystallinity of the cellulose, for a biofuels study.
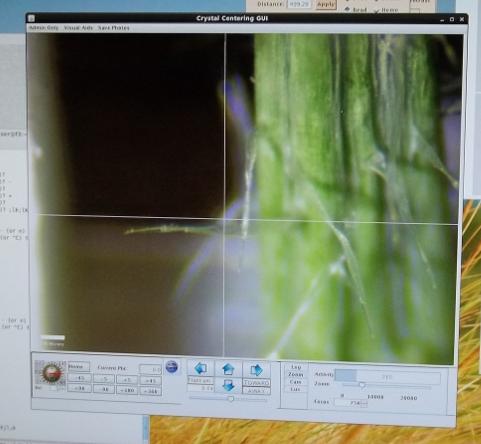
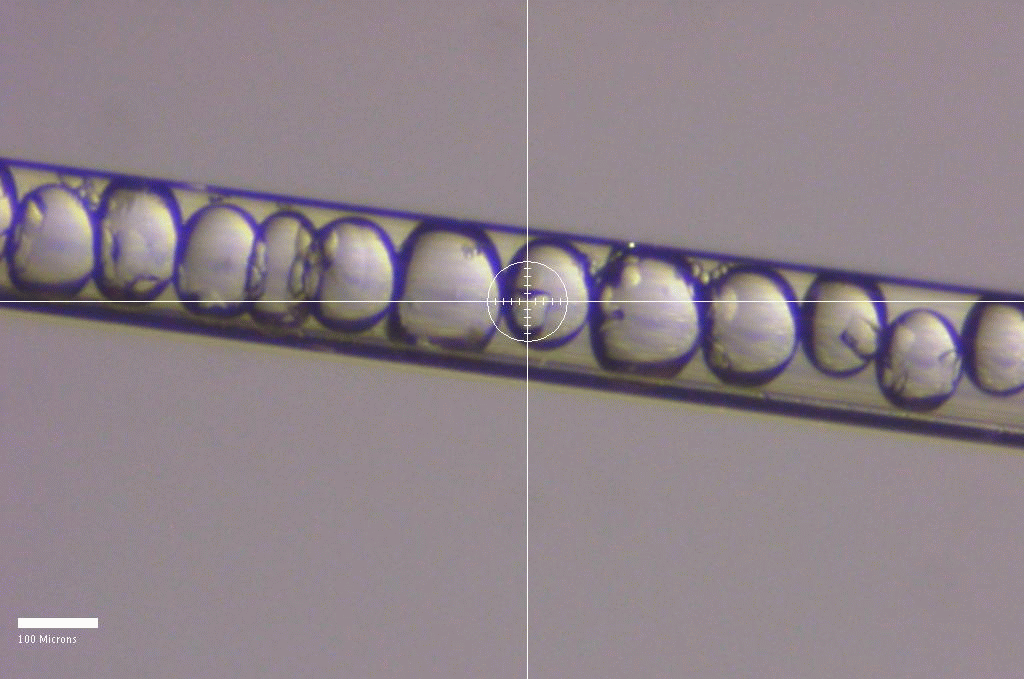
Diffraction from crystals in droplets in a capillary, as part of a collaborative serial crystallography project.
Monitoring of radiation damage using Laue diffraction.