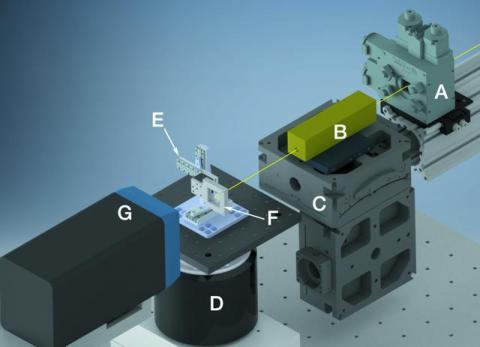
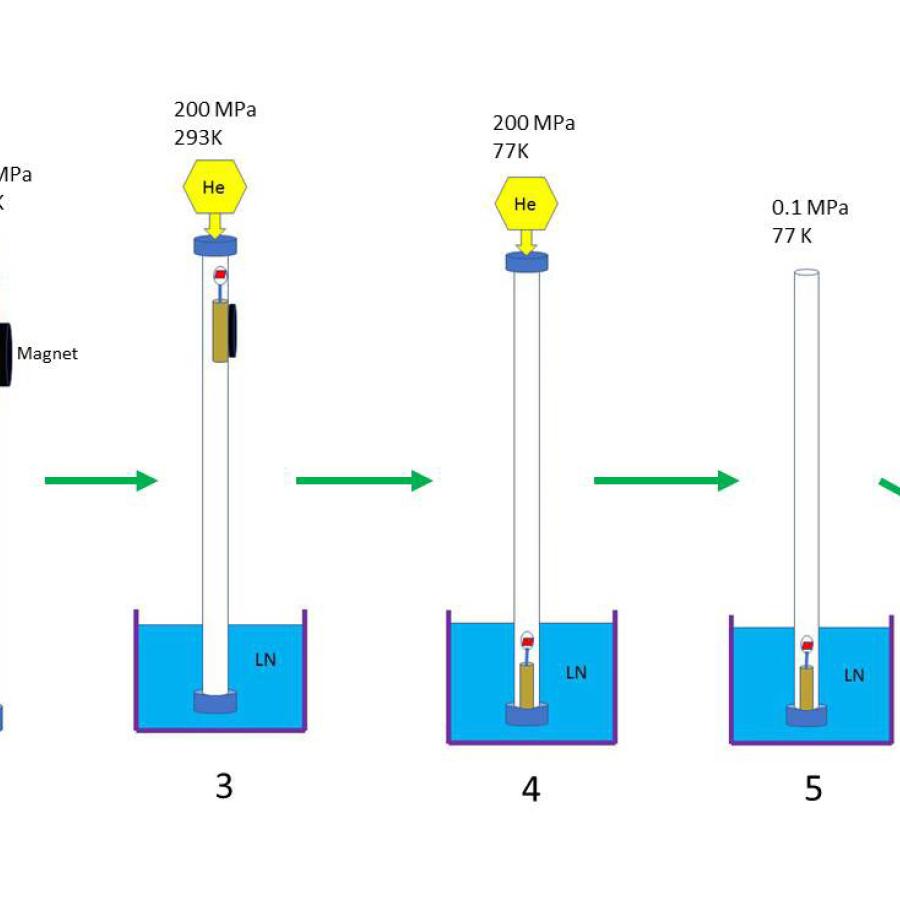
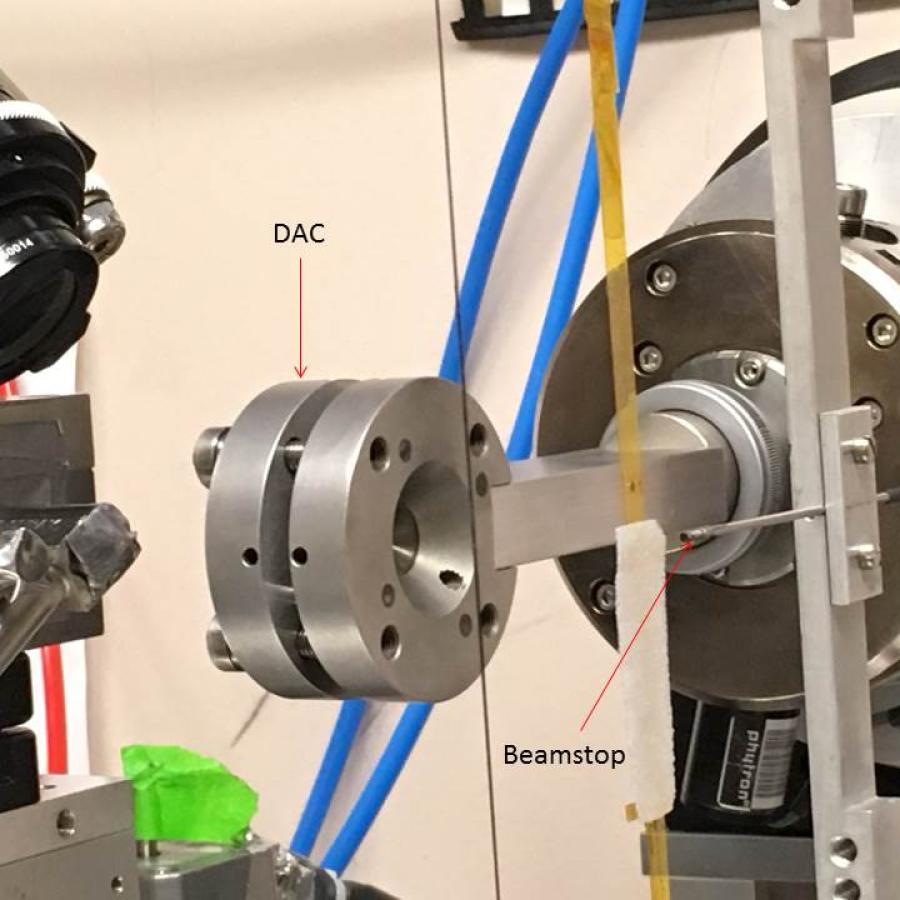
One major bottleneck in the protein crystallography experiment is collection of a complete dataset prior to the onset of radiation damage. In certain cases, such as with microcrystalline samples, or data collection at room temperature, complete data sets are most efficiently obtained by collecting partial datasets from many crystals. With improved microbeam performance, novel techniques in sample delivery, and advances in post-processing of partial data sets, MacCHESS offers users an advanced serial microcrystallography experience.
There are many cases in macromolecular crystallography where crystals cannot be grown to a size sufficient to collect a full dataset for structural analysis, due in part to rapid onset of radiation damage. In such cases, where microcrystal formation dominates the crystallization landscape, much effort has been undertaken to use the microcrystals that are available to their fullest extent. To that end, the technique of serial crystallography- the collection of a series of small datasets of partial completeness from many crystals- was developed. In this technique, a small dataset is collected from each crystal, and the collection of partial data is evaluated and merged together to generate a complete dataset. Collecting crystal datasets serially is preferable in cases where the small size of the crystals is not the limiting factor, e.g. where diffraction data can be more accurately measured from multiple crystals than from a single crystal, due to reduced radiation damage to each crystal and increased data multiplicity. We are developing methodologies for streamlining serial crystallography, so that the technique can be made available for any user.