Sidebar Menu (View Pages)
- Status
- ⌃ Science
-
⌃
Users
- What's the process? - Prospective User Guide
- User Guide
- Beamline Directory
- CHESS Deadlines
- X-Ray Run Schedule
- CHESS 2026_1 Updates
- Shipping
- ⌃ Safety
- Travel and Lodging
- Acknowledgments
- User Agreement
- CHESS Status Page
- ⌃ Technical Resources
- ⌃ Facilities
- ⌃ Public
- Industry
- ⌃ About
Tags
Featured
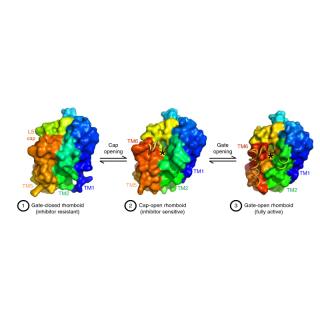
Step-by-Step: Revealing the mechanism of a protein-cleaving enzyme by crystallographic snapshots
"These unusual enzymes have been implicated in diseases ranging from Alzheimer’s disease to malaria, type II diabetes, cancer, Parkinson’s disease, cholera and tuberculosis."

A Winning CHESS Opening
This October, the new user facilities at the Cornell High-Energy Synchrotron Source (CHESS) will open their doors to researchers. This opening follows a major upgrade project, known as CHESS-U, which establishes CHESS as one of the world’s leading X-ray sources.

NSF Delegation visits CHESS
Last month, Linda Sapochack, NSF Division Director for the Division of Materials Research (DMR), Clark Cooper, Senior Advisor for Science and Head of the Office of Multidisciplinary Activities in the NSF Directorate for Mathematical and Physical Sciences, and NSF Program Director Guebre X. Tessema visited CHESS to see the newly upgraded facility.

NIH awards $17.4 million to Cornell for CHESS subfacility
A single human cell contains thousands of proteins that perform a vast array of functions, from fighting off viruses to transcribing DNA. By understanding the structure of these proteins, researchers can interpret their functions and develop methods for turning them on and off.

2019 CHESS Users' Meeting and Workshops
"The CHESS Users’ Meeting attracted a record number of 225 registered participants to the Cornell campus to look back at major milestones of the project and to discuss X-ray science enabled by the ambitious upgrade."
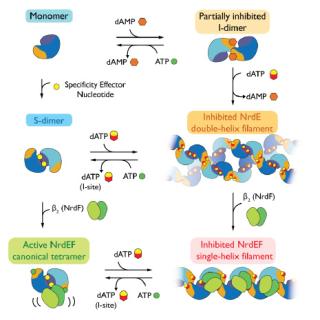
Conformational Gymnastics Necessary for Ribonucleotide Reductase Activity
"By understanding how an essential enzyme is inactivated in an organism-specific manner, the researchers hope to contribute to the development of new anti-pathogenetic therapies."
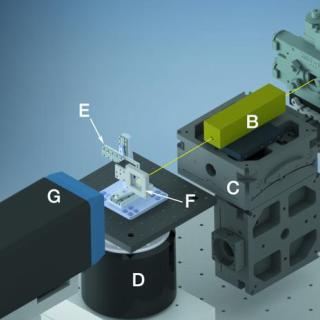
Room temperature serial oscillation crystallography
Serial crystallography is a method for obtaining structural information on an atomic level of a protein, without the need for large protein crystals. Instead, small diffraction datasets are collected on many small protein crystals, which are usually easier to obtain than large ones. Serial crystallography is an ideal method for collecting diffraction data of proteins at room temperature, where the onset of radiation damage from the X-ray beam is rapid.

Study offers new target for antibiotic resistant bacteria
As antibiotic resistance rises, the search for new antibiotic strategies has become imperative. In 2013, the Centers for Disease Control estimated that antibiotic resistant bacteria cause at least 2 million infections and 23,000 deaths a year in the U.S.; a recent report raised the likely mortality rate to 162,044.