However, students from the groups of Brian Korgel and Tom Truskett at the University of Texas at Austin, found just the opposite behavior in superlattices of small gold nanoparticles [1]. These consist of a 2 nm gold core surrounded by organic octodecane thiol ligands with about the same length. Upon casting from solution, these particles form superlattices with a BCC structure [2]. Similar to block copolymer micelles with sufficiently long ligands the BCC lattice is favored over the denser FCC lattice, as the entropy of the ligands is maximized [3,4].
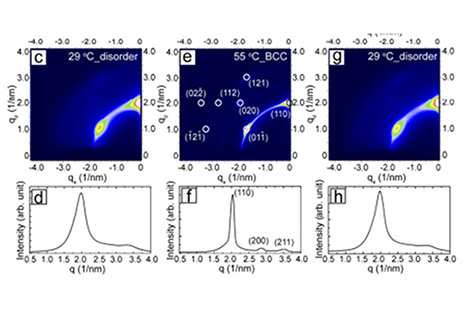
Yixuan Yu expertly synthesized the particles, and with the help of fellow students Avni Jain, Vikas Reddy and Adrien Guillaussier characterized the particle assemblies with GISAXS at CHESS beamline D1. Staff scientist Detlef Smilgies provided a heating stage where samples could be studied between room temperature and 300°C [5]. At room temperature the particles already displayed a nice BCC superlattice. It has been previously known that upon heating the superlattice reflections sharpen up, until a chemical reaction sets in above 100°C [2]. Avoiding the reaction threshold by limiting the temperature range to 55°C, particles could be cooled back to room temperature – and disordered again. And this over many cycles! This behavior is different from thermal annealing: usually a material with kinetically limited ordering can be improved by heating it close to the glass temperature. As the ordered state is closer to the equilibrium structure, it is maintained upon cooling.
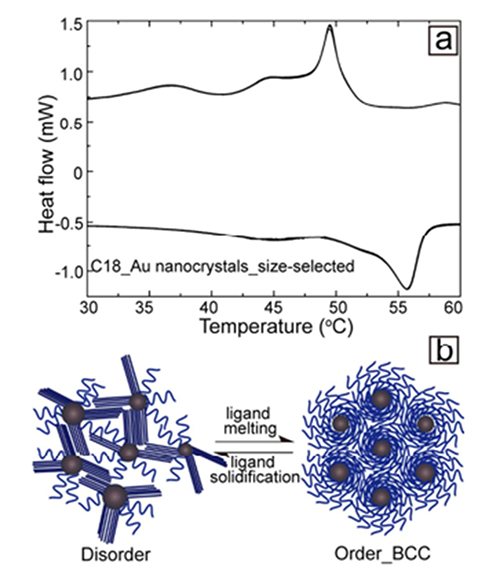
Yu and fellow students embarked on detective work to find the origin of this unusual behavior of their particles which has been called “reverse melting”. Differential scanning calorimetry (DSC) finally gave it away: the alkane chains of the octodecane thiol ligand undergo a melting transition above 50°C. In the more liquid form ligands can accommodate highly ordered superlattices. Upon cooling ligands stiffen up again, and the superlattice order is compromised. The study demonstrates that complex materials can provide anomalous thermal behavior due to phase changes in one of their components.
References:
[1] Yixuan Yu, Avni Jain, Adrien Guillaussier, Vikas Reddy, Thomas M Truskett, Detlef Smilgies, Brian A Korgel, "Nanocrystal Superlattices that Exhibit Improved Order On Heating: An Example of Inverse Melting?", Faraday Discussions, DOI: 10.1039/C5FD00006H (online 2015).
[2] Brian W. Goodfellow, Michael R. Rasch, Colin M. Hessel, Reken N. Patel, Detlef-M. Smilgies, and Brian A. Korgel, "Ordered Structure Rearrangements In Heated Gold Nanocrystal Superlattices", Nano Lett. 13, 5710–5714 (2013).
[3] Alice P. Gast and William B. Russel, "Simple Ordering in Complex Fluids", Physics Today (December 1998), 24-30.
[4] Brian W. Goodfellow and Brian A. Korgel, "Reversible Solvent Vapor-Mediated Phase Changes in Nanocrystal Superlattices", ACS Nano 5, 2419–2424 (2011).
[5] http://news.chess.cornell.edu/articles/2014/Smilgies140328.html