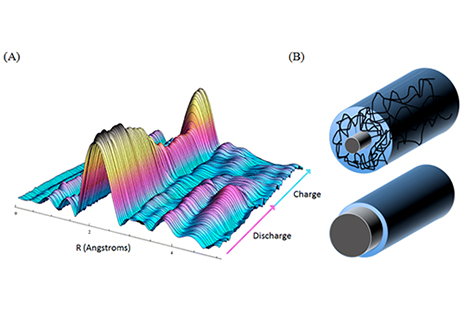
Most commercial rechargeable batteries are based on lithium ion intercalation into layered metal oxides, the mechanism of which is fairly well understood. To move forward in the development of better electrode materials, deeper insights into heretofore unexplored methods of charge storage must be gained. Synchrotron-based techniques such as X-ray absorption spectroscopy (XAS) and X-ray diffraction (XRD) are invaluable tools to study molecules, materials, and systems relevant to electrochemical energy storage since they can provide structural and compositional information under realistic operating conditions (operando studies). A specially designed coin cell allows for the operando investigation of structural, compositional, and chemical changes within the battery as a function of the state of charge.
At Cornell University, researchers in the Abruña Electrochemistry Lab have explored the use of germanium nanowires grown in the Hanrath Energy Lab as anodes for LIBs. In a newly published article, lead author Katharine Silberstein (a CHESS-affiliated graduate student in Chemistry and Chemical Biology) and her collaborators studied the nanowires’ chemical structural transformations by XAS and XRD at CHESS in real time during battery cycling. It was discovered that lithiation occurs heterogeneously, preferentially into amorphous regions over crystalline domains, to the Ge4Li15 alloy phase. Those crystalline domains could be preserved for a few cycles if the voltage cutoff was above the point of full lithiation. This level of detailed understanding is critical to advance designs for next-generation anode materials.
Details of this new research can be found in:
Katharine E. Silberstein, Michael A. Lowe, Benjamin Richards, Jie Gao, Tobias Hanrath, and Hector D. Abruna, “Operando X-ray Scattering and Spectroscopic Analysis of Germanium Nanowire Anodes in Lithium Ion Batteries”, Langmuir 31(6), 2028–2035 DOI:10.1021/la504382q (2015).