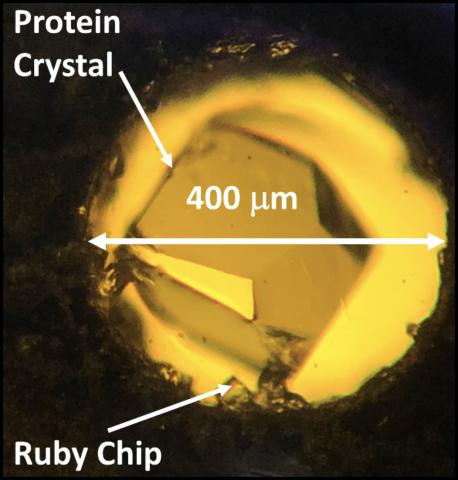
There are many questions about the behavior of proteins under pressure that have not been experimentally resolved. Some very simple but important examples are: how does a protein crystal’s structure change as a function of pressure, and how large an external pressure can be sustained by the crystal? To answer such questions, X-ray crystallography is the obvious method of choice, but requires the use of a pressure cell capable of precise control of pressure, with small steps between successive pressures. Construction of an experimental system incorporating such a cell is challenging. A group of scientists working at The CHESS FlexX beamline jointly supported by NSF, NIH and NYSTAR succeeded in collecting crystallographic data from a crystal of the protein beta-lactoglobulin mounted in a DAC with a resolution of 2.0 Å (Figure below). Surprisingly, the protein crystal can survive under pressure as high as 8900 bar, one order of magnitude greater than the pressure at the bottom of the ocean (i.e. 11000 m). Upon data processing and analysis, the group found that the crystal displays strong anisotropic compression and diffracts well under pressure.
Broader Impacts
Much of the biosphere of the Earth exists at pressures over a range of 100 bar to several kilobar, but it remains very little known how pressure affects the organisms and thus impacts the biological activities within this environment. This work reveals two significant aspects of pressure effects: 1) pressure reduces the volume of biomolecule crystals anisotropically and may improve the diffraction properties; and 2) the biomolecule crystals tested, unexpectedly, tolerate substantial pressure increases without apparent damage. It turns out that there are rich physics and novel phenomena behind a variety of biomolecules under pressurization. This work will certainly attract scientists from various communities to explore the behaviors and underlying mechanism of biomolecules under pressure.
Need for CHEXS Experimental Capabilities
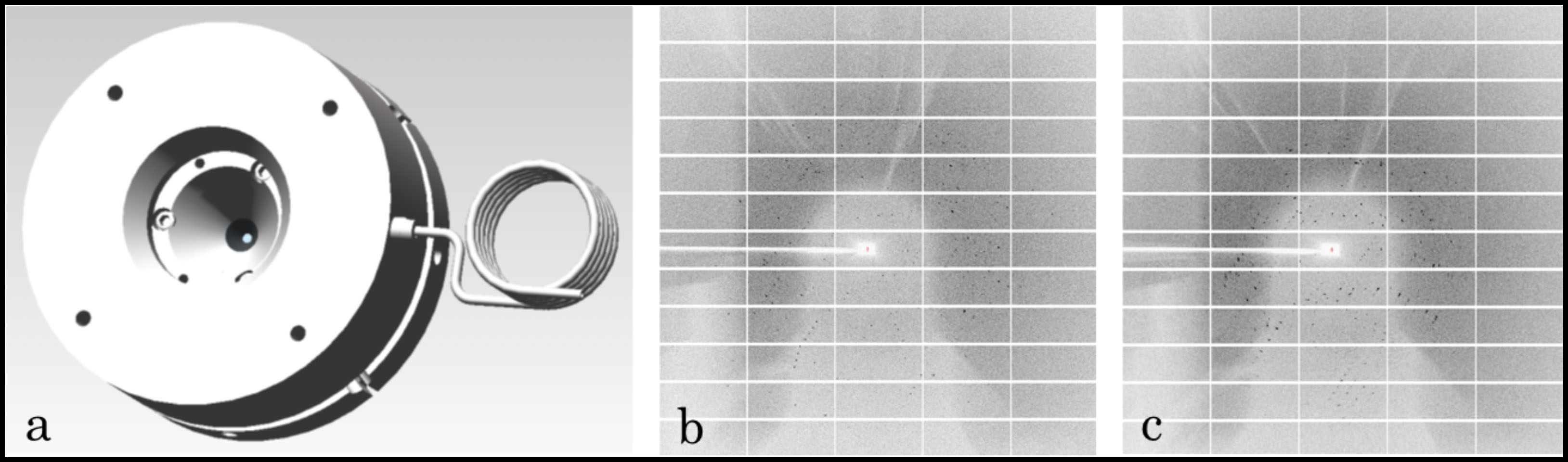
CHESS has always led efforts in building new instrumentation to address the questions of newly emergent science frontiers. In order to explore the behavior of a variety of biomacromolecules under pressure, CHESS led a team effort and built the unique DAC-based macromolecule crystallography instrumentation which works typically on biological samples, and does not exist at other U.S. synchrotron sources. The crystallographic datasets were collected in-situ under pressure on the FlexX beamline using a gas-driven vivoDAC and a Pilatus 6M detector. Taking advantage of the excellent tuning ability of precise pressure and the large angular opening of the DAC, the scientists were able to make significant progress towards collecting full crystallographic data sets that will reveal the structure of biomolecular crystals as a function of pressure, and promote understanding of the underlying biophysical mechanisms.
Collaborators:
Zhongwu Wang, Xin Huang, Durgesh Rai, Aaron Finke, Marian Szebenyi, and Qingqiu Huang,
CHESS, Cornell University, Ithaca, NY, 14850, USA
Sol Gruner, CHESS & Physics Department, Cornell University, Ithaca, NY, 14850, USA
How was the work funded?
CHEXS is supported by NSF award DMR-1829070, and the MacCHESS facility is supported by NIH/NIGMS Grants GM-103485 and GM-124166.