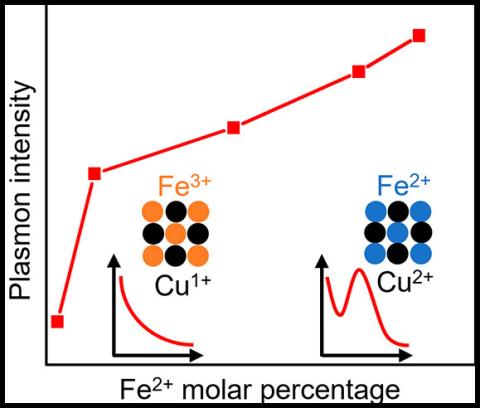
Plasmonic semiconductor nanocrystals have become an appealing avenue for researching nanoscale plasmonic effects due to their wide spectral range (visible to infrared) and great tunability compared to traditional precious metal nanocrystals. CuFeS2 is an exciting semiconductor that has a prominent plasmon absorption band in the visible range (∼498 nm). In this work, the researchers determined the origin of the plasmonic behavior in CuFeS2 by characterizing the nucleation and growth stages of the reaction through a series of ex situ and in situ probes (e.g., X-ray absorption spectroscopy and X-ray emission spectroscopy). They showed that the plasmon formation is driven by band structure modification from Fe(II) incorporation into the nanocrystals. Mixed oxidation state of Cu(I)/Cu(II) and Fe(II)/Fe(III) was observed. Using these results, the researchers proposed a reaction mechanism for synthesis of CuFeS2 and outlined a method to verify the phase purity of the material.
What are the broader impacts of this work?
Not only did this work allow the authors to propose a mechanism of the synthesis of CuFeS2, but it also provided a theoretical basis for tuning plasmon intensity by changing the cation oxidation state by varying synthesis conditions. Overall, this work on CuFeS2 serves as a model for studying intermediate band ternary plasmonic nanoclusters.
Need for CHEXS Experimental Capabilities
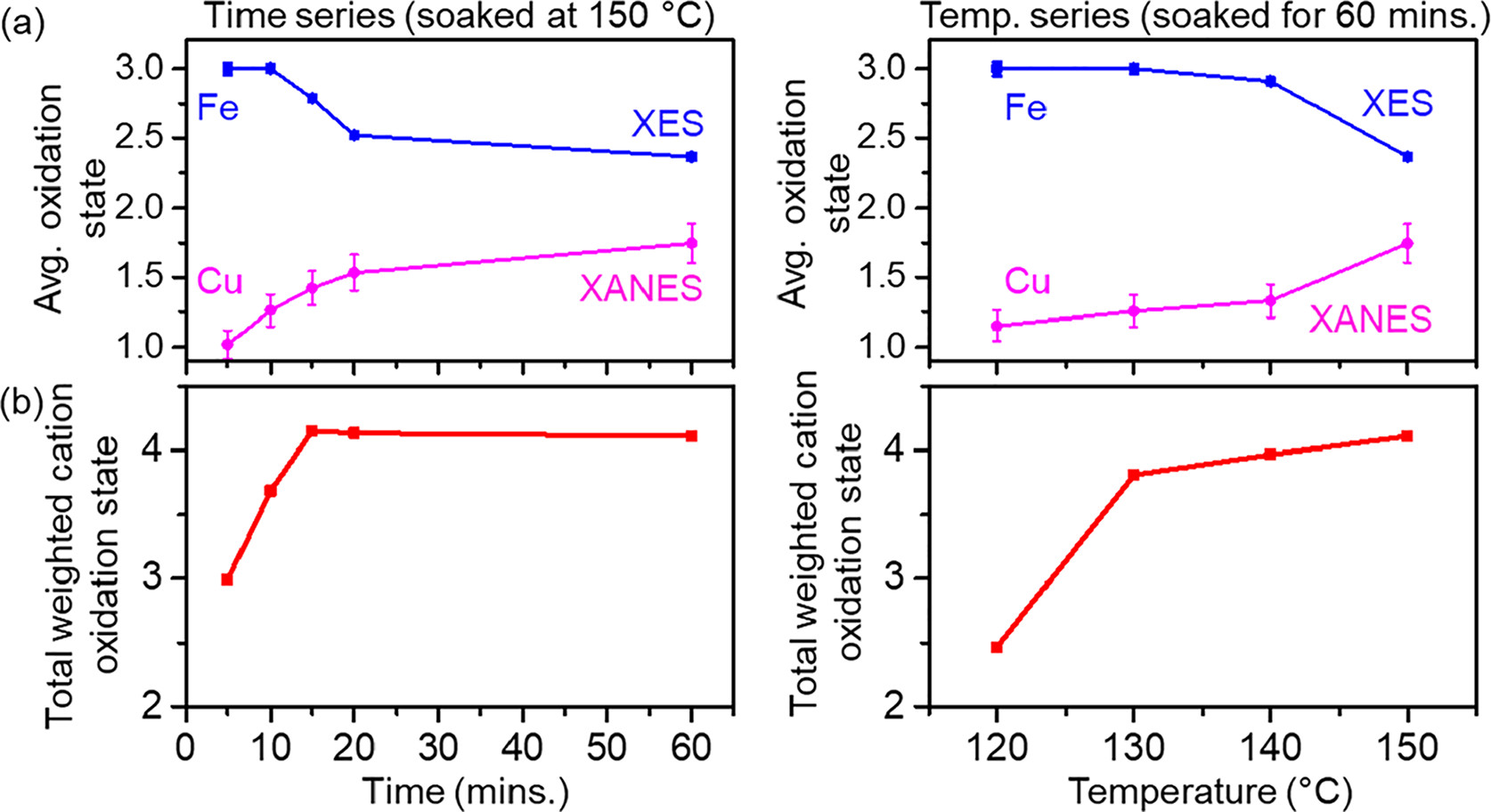
This work made use of the high intensity x-rays and x-ray emission spectrometer available at the PIPOXS beamline to perform ex situ and in situ x-ray absorption and x-ray emission spectroscopic measurements.
Funding
This work was supported in part by the National Science Foundation (NSF) under award numbers DMR-2003431, CHE-1665305, and CHE-1507753. The XES experiments made use of the Cornell Centre for Materials Research (CCMR), and Cornell High Energy X-ray Sciences (CHEXS) facilities funded in part by the National Science Foundation under award numbers DMR-1719875 and DMR-1829070. The X-ray absorption spectroscopy experiments were performed using beamline 7-BM (QAS) of the National Synchrotron Light Source II, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704.
References
Fe Cations Control the Plasmon Evolution in CuFeS2 Nanocrystals
Yuan Yao, Anuj Bhargava, and Richard D. Robinson
Chemistry of Materials 2021 33 (2), 608-615; doi.org/10.1021/acs.