Lower-pressure cryocooling system: pressure usually used is 5-20 MPa, for gas trapping:
a) Gas-loading and Cryo-cooling apparatus and b) Schematic overall view.
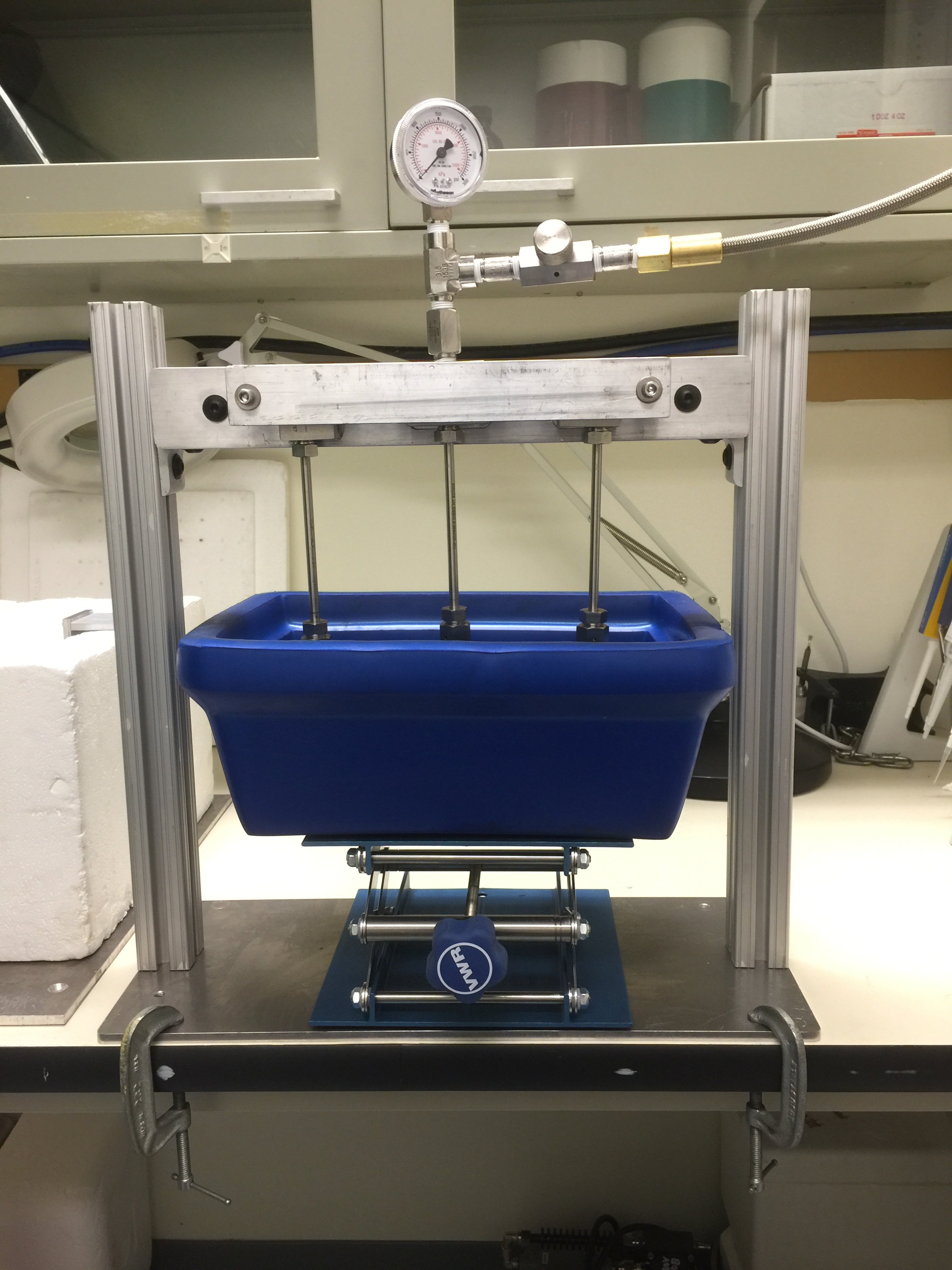
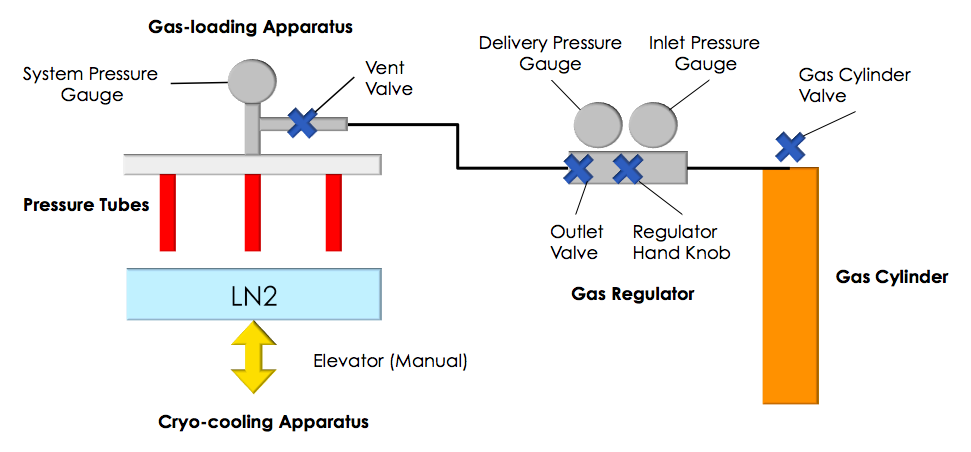
A pressure cryo-cooling system is built to safely handle biological gases, such as carbon dioxide and oxygen, including hazardous gases such as nitric oxide.
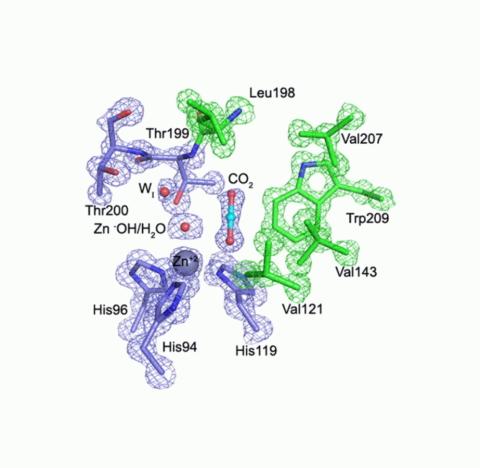
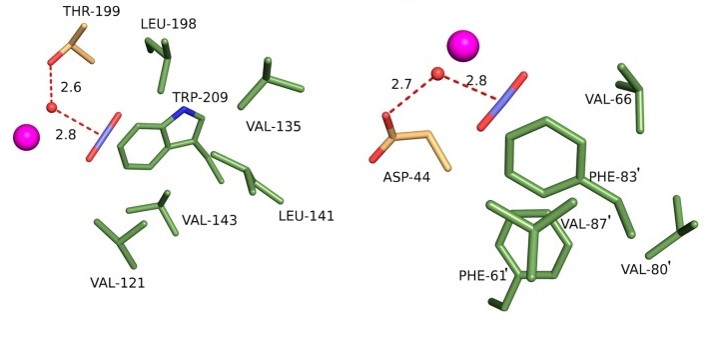
An example: Carbonic anhydrase (CA) regulates the pH of the blood by converting carbon dioxide to bicarbonate. There was a model for how the enzyme does this, but pressurizing human CA II with CO2, at a pressure of 15 atmospheres, and cryocooling, let researchers actually see where the CO2 goes and confirm the model (1). Later work allowed comparison of CO2 binding in bacterial and human CAs (2).
References:
(1) Domsic et al. 2008, J. Biol. Chem. 45, 30766-30771
(2) Aggarwal et al. 2015, Biochemistry 54, 6631-6638