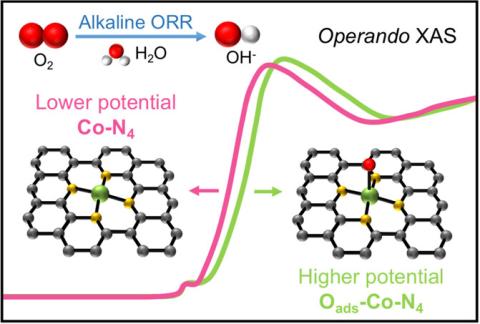
Now, in a new paper appearing in the Journal of the American Chemical Society, a team of researchers from Cornell and the University of Wisconsin report new catalysts which exhibit superior ORR activity and robust stability. The team has characterized metal–organic framework-derived nonprecious dual metal single-atom catalysts (SACs), consisting of Co–N4 and Zn–N4 local structures. Their remarkable performance was validated under realistic fuel cell working conditions, achieving a record-high peak power density of ∼1 W cm–2 among the reported SACs for alkaline fuel cells. Operando X-ray absorption spectroscopy studies at the PIPOXS beamline at CHEXS revealed that the Co atom in the Co–N4 structure is the main catalytically active center. This work provides a comprehensive mechanistic understanding of the active sites in the Zn/Co–N–C catalysts and will pave the way for the future design and advancement of high-performance single-site electrocatalysts for fuel cells and other energy applications.
Why is this important?
Hydrogen fuel cells are high-efficiency regenerative energy-conversion devices for electric vehicles, portable electronic devices, and stationary applications. However, accelerating the sluggish kinetics of the oxygen reduction reaction traditionally requires significant amounts of expensive platinum-based catalysts in proton exchange membrane fuel cells. The high catalyst cost, up to 40% of the total cost, has hindered large-scale application and deployment of these fuel cells. Single-atom catalysts (SACs) are an emerging alternative where isolated nonprecious metal atoms anchored on a supporting substrate are the metal active centers. The Cornell/Wisconsin team has now identified, characterized, and understood a record-setting new SAC material, using cobalt atoms dispersed on metal-organic-framework scaffold. Their findings provide insight into the nature of the active site in the material and the dynamic evolution under electrocatalytic conditions, which will help to pave the way for developing high-performance electrocatalysts for renewable energy applications.
Why did this research need CHEXS?
The PIPOXS beamline at CHEXS is dedicated to solving challenging chemical problems related to operando catalysis, batteries, and fuel cells using cutting-edge x-ray spectroscopy. This research used PIPOXS both for ex-situ EXAFS studies to characterize the Co and Zn environments in the newly synthesized catalyst materials and also operando EXAFS and XANES measurements of the catalysts inside operating fuel cells in order to understand catalyst function. These operando measurements were critical for identifying Co as the active site. The high flux, high resolution doubly-focused undulator beams available at PIPOXS are precisely and rapidly energy tunable to access the edges of 74 different elements, including the K-edges of the 3d transition metals relevant for this work.
How was the work funded?
The Center for High Energy X-ray Sciences (CHEXS), NSF MPS/BIO/ENG (DMR-1829070)
Center for Alkaline Based Energy Solutions (CABES), an Energy Frontier Research Center (EFRC) supported by the US DoE under Grant DE-SC-0019445
Cornell Center for Materials Research (CCMR), NSF MRSEC program (DMR-1719875)
National Energy Research Scientific Computing Center, a DOE Office of Science User Facility, (contract no. DE-AC02-05CH11231 / NERSC BES-ERCAP0022773)
Reference
Atomically Dispersed Zn/Co–N–C as ORR Electrocatalysts for Alkaline Fuel Cells
Weixuan Xu, Rui Zeng, Michael Rebarchik, Alvaro Posada-Borbón, Huiqi Li, Christopher J Pollock, Manos Mavrikakis, and Héctor D Abruña
J. Am. Chem. Soc. (2024), 146, 4, 2593–2603; https://doi.org/10.1021/jacs.3c11355