What is the discovery?
Scientists have developed an innovative methodology for studying compounds containing precious heavy metals, like platinum, with potentially significant implications for catalysis research. This approach involves using resonant excitation to dramatically improve the resolution of x-ray emission spectra (XES), revealing chemical insights that were previously unattainable.
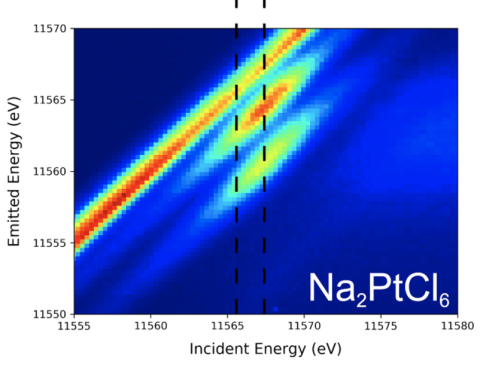
When applied to heavy metals, conventional (i.e. non-resonant) XES experiments often yield broad and featureless spectra, preventing these data from revealing information about systems containing these metals. By shifting the incident energy to a specific absorption band of the heavy metal, scientists were able to dramatically improve the resolution of the XES spectra, unveiling previously hidden details about the chemical environment and bonding of heavy metals in compounds. This improvement in resolution enabled even closely related chemical compounds to be distinguished and thus establishes resonant XES as a viable probe of chemically complex samples of heavy metals compounds.
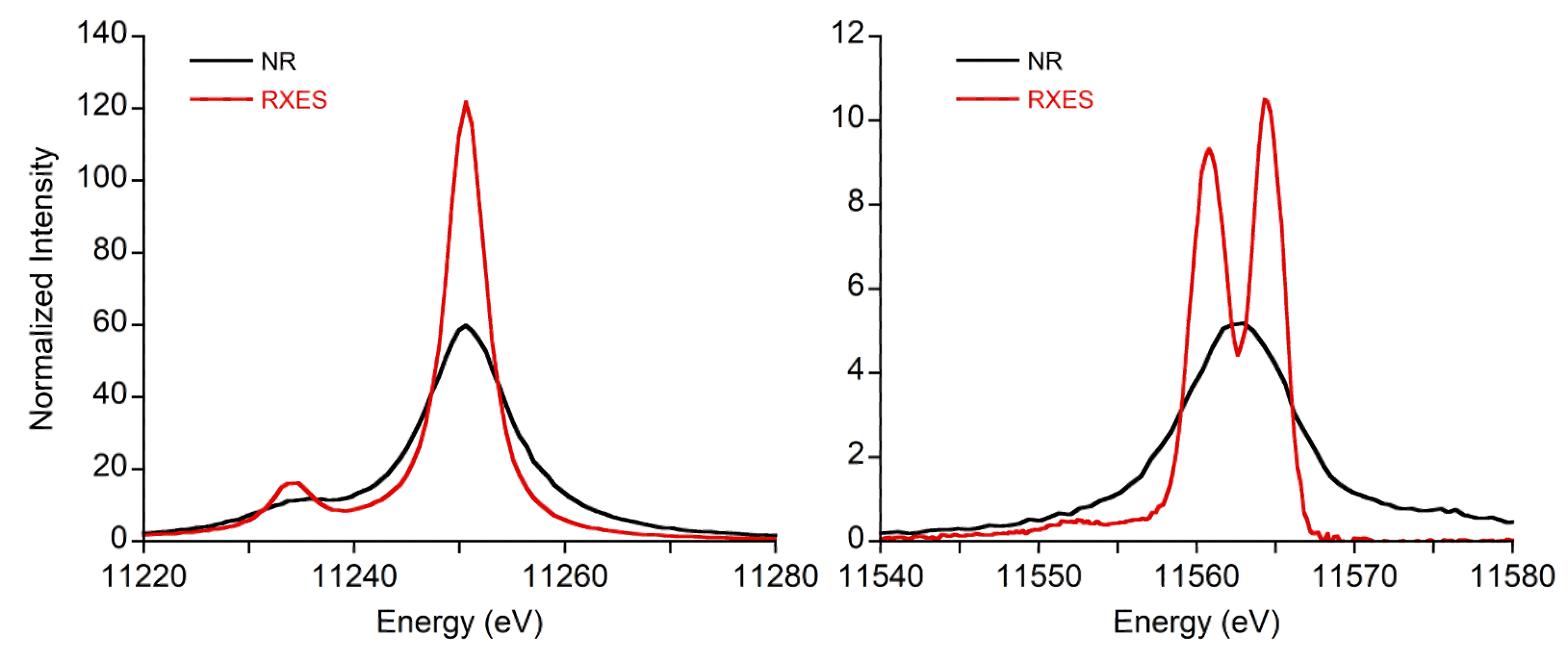
Top: An overlay of non-resonant X-ray emission spectroscopy (NRXES) and resonant X-ray emission spectroscopy (RXES) spectra for Na2PtCl6 showing the dramatic spectral sharpening in the mainline (left) and the valence-to-core (VtC) regions (right).
Why is it important
Heavy metals, such as platinum, are valued as catalysts due to their ability to perform challenging two-electron chemical reactions. Unlike lighter metals that often lead to damaging radical reactions, heavy metals offer precise control and efficient catalytic activity. Platinum, in particular, holds immense significance as a catalyst for vital processes like water splitting, a cornerstone of clean energy production. However, heavy metals are scarce and expensive, necessitating their optimized use. The ability to gain detailed insights into the chemical environment, oxidation states, and bonding interactions of heavy metals offers the ability for precise understanding of how heavy metals perform their chemistry, thus opening the door for the rational design of more active and efficient catalysts.
Why did this research need CHESS?
Beyond simply applying x-ray methods to answer questions in domain science, CHESS is also committed to developing entirely new methodologies for studying complex systems. This commitment allows users the freedom to explore unconventional approaches, which in turn can open up x-ray methods to new and unserved communities. By combining this intellectual freedom with the unique technical capabilities available at CHESS—including the dual array valence emission spectrometer (DAVES) and intense, energy-tunable x-ray source—scientists now have a cutting-edge tool to investigate heavy metals, leading to insights that could revolutionize catalysis and contribute to the development of more sustainable and efficient energy technologies.
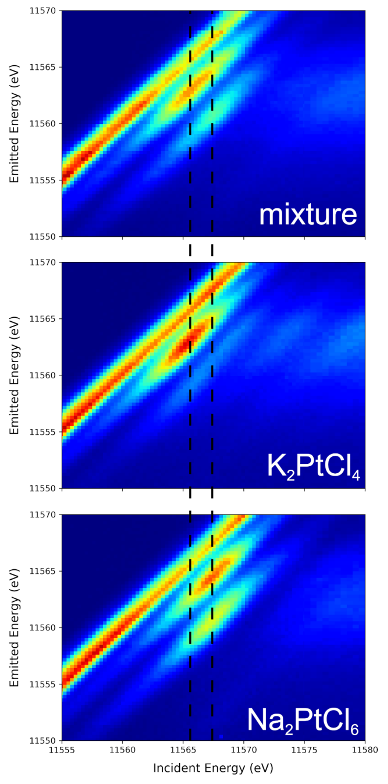
Top: A VtC RXES plane of a ~1:1 molar ratio mixture of K2PtCl4 and Na2PtCl6 (top) is shown, along with planes for pure K2PtCl4 (middle) and Na2PtCl6 (bottom). Guidelines through the planes at constant incident energies corresponding to absorption maxima for K2PtCl4 (left dashed vertical line) and for Na2PtCl6 (right vertical line) demonstrate both individual components give rise to resolved features in the data of the mixture. The intense feature where incident and emitted energy are equal is the scattered beam.
How was the work funded?
This work is based on research conducted at the Center for High-Energy X-ray Sciences (CHEXS), which is supported by the at National Science Foundation (BIO, ENG and MPS Directorates) under award DMR-1829070. This work was also support by NSF-PREM: Center for Interfacial Electrochemistry of Energy Materials (CiE2M) under award DMR- 1827622.