What is the discovery?
Increasing demand for electric vehicles and energy storage devices necessitates the development of cost-effective and energy-dense lithium-ion batteries. Cathode materials which contain Ni, Mn, and Co (aka the NMC cathodes) are the current state-of-the-art. Concerns about supply chains and the volatility of prices for Ni and Co, combined with environmental concerns linked to the mining and processing of these metals, motivate a search for alternative cathode materials using earth-abundant elements. Significant effort has been devoted to finding Co-free, Mn- and Fe-rich cathode materials, however these have been plagued by poor electrochemical performance and poor cycling stability - the reasons for which remain unclear. Now, in a new paper appearing in the journal “Chemistry of Materials”, a group of researchers from Oak Ridge National Laboratory, Argonne National Laboratory, and CHEXS use high-energy x-ray spectroscopy to gain fundamental insights into the redox processes and migration tendencies of transition metals in the cathode material 0.7(Li2MnO3)·0.3(LiFeO2). This work improves understanding of the Fe and Mn oxidation states and local structural changes due to metal migration before and after the activation process. It specifically and unambiguously correlates the oxidation and spin-state of Fe with the discharge state of the cathode material, exploiting the spin-selectivity of HERFD-XANES measurements at the PIPOXS beamline at CHEXS in different stages of the charge-discharge cycle.
Why is this important?
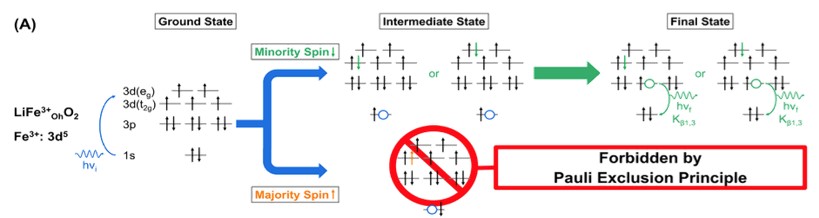
This research constitutes the first utilization of spin selective XANES to provide unequivocal evidence on the intricate relationships between the transition metals’ oxidation states, local structure, and electrochemistry of a cathode material. By gaining specific understanding of the spin state, oxidation state, and redox activity of Fe and Mn throughout each stage of the electrochemical cycle, the team of CHEXS users is able to correlate local chemistry with specific cathode properties, including the irreversible discharge capacity during initial cycling, considerable structural distortions during continual Li extraction, and the eventual development of cycling instability due to distortions in Mn-rich domains. These material deficiencies must be first understood in order to be overcome. The precision insight offered by x-ray spectroscopy is necessary to engineer more stable and effective earth-abundant cathode materials for the future.
Why did this research need CHEXS?
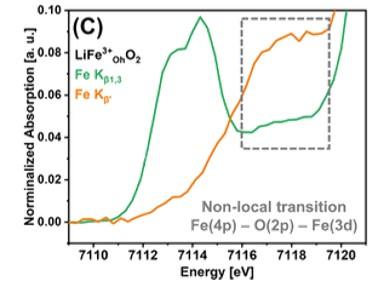
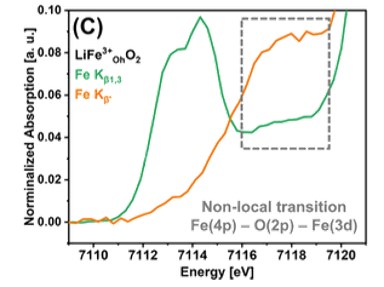
The PIPOXS beamline at CHEXS is dedicated to solving challenging chemical problems related to operando catalysis, batteries, and fuel cells, using cutting-edge x-ray spectroscopy. This research made use of the unique DAVES spectrometer at PIPOXS, which combines high-resolution final-energy selection with a large solid angle for data collection and can resolve extremely weak resonant emission signals. The high flux, high resolution doubly-focused undulator beams available at PIPOXS are precisely and rapidly energy tunable to access the edges of 74 different elements, including the K-edges of the 3d transition metals relevant for earth-abundant catalysis. 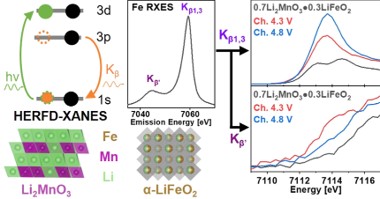 Together, the high incident and emitted energy resolutions were combined to probe the very weak resonantly-excited pre-edge features of these cathode materials.
Together, the high incident and emitted energy resolutions were combined to probe the very weak resonantly-excited pre-edge features of these cathode materials.
How was the work funded?
The Center for High Energy X-ray Sciences (CHEXS), NSF MPS/BIO/ENG (DMR-1829070)
Vehicle Technologies Office, US DOE (Earth-Abundant Cathode Active Materials Consortium)
ORNL, US DOE, DE-AC05-00OR22725
ANL, US DOE, DE-AC02-06CH11357
Reference
Redox Mechanisms and Migration Tendencies in Earth-Abundant 0.7Li2MnO3·0.3LiFeO2 Cathodes: Coupling Spin-Resolved X‐ray Absorption Near Edge and X‐ray Absorption Fine Structure Spectroscopies
CY Kwok, S Mallick, CJ Pollock, A Gutierrez, M Dixit, JR Croy, and M Balasubramanian
Chem. Mater. 2024, 36, 1, 300–312; https://doi.org/10.1021/acs.chemmater.3c02111