What is the discovery?
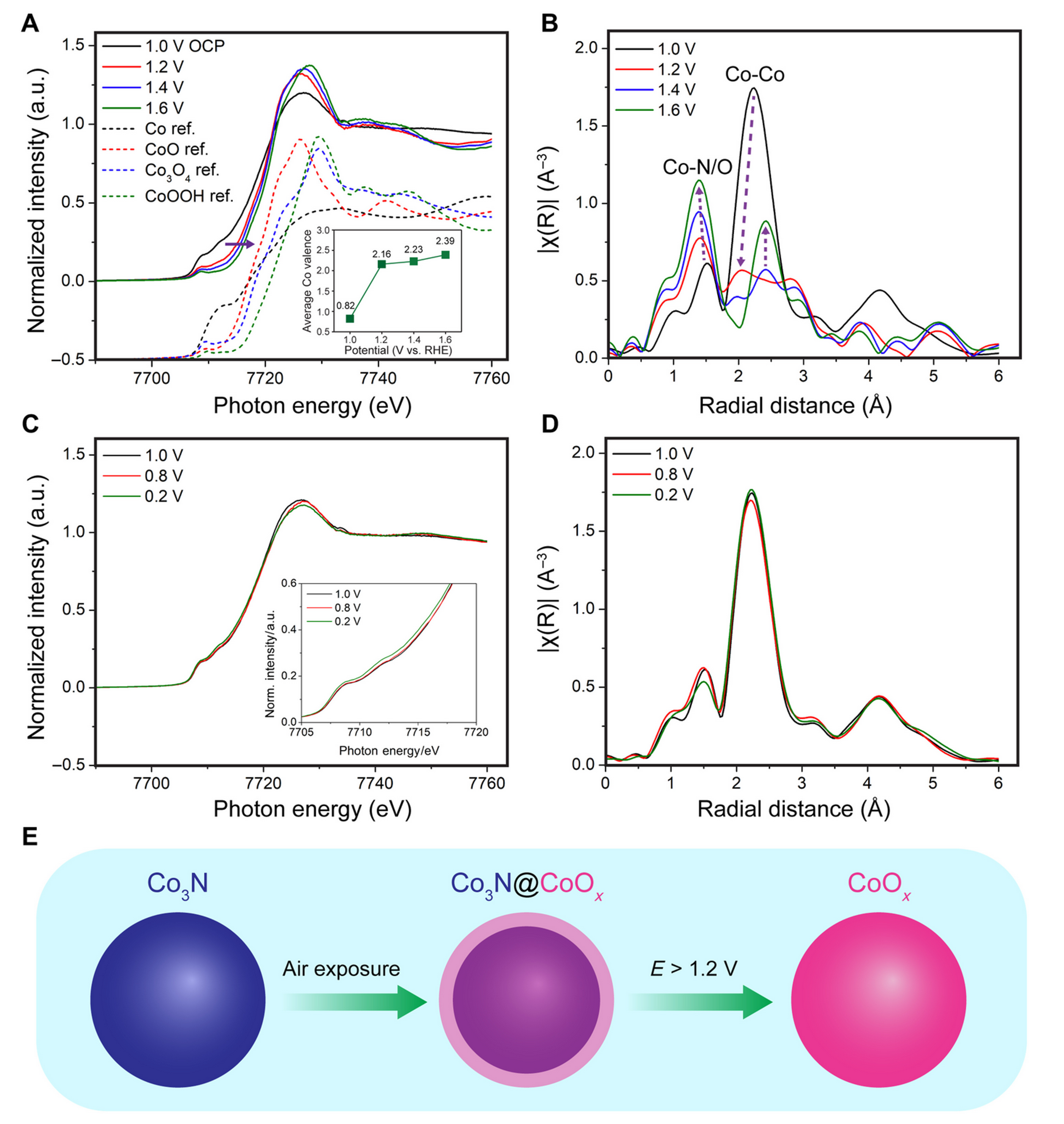
CHEXS users have discovered a class of nonprecious metal derivatives that can catalyze fuel cell reactions about as well as platinum, at a fraction of the cost. A critical part of the fuel cell is the oxygen reduction reaction, an infamously sluggish process that is traditionally sped up by platinum and other precious metals. Now, in a new paper appearing in the journal Science Advances, a team lead by Héctor Abruña (the Émile M. Chamot Professor of Chemistry and Chemical Biology at Cornell University), have reported a new cobalt nitride catalyst material with near identical efficiency to platinum while costing 475 times less (as of February 2022). Carbon-supported cobalt nitride (Co3N/C) achieved a record-high peak power density among reported nitride cathode catalysts of 700 mW cm−2 in alkaline membrane electrode assemblies. The material was demonstrated to remain stable below 1.0V potentials inside working fuel cells, using operando x-ray spectroscopy at the PIPOXS beamline. Operando XANES and EXAFS (A,B) show dramatic changes in valence and bond lengths for potentials above 1V, while below 1V the material remains stable (C,D).
Why is it important?
This finding brings closer a future where hydrogen fuel cells efficiently power cars, generators and even spacecraft with minimal greenhouse gas emissions. Less expensive metals will enable wider deployment of hydrogen fuel cells. They will push us away from fossil fuels and toward renewable energy sources.
Why did this research need CHEXS?
The PIPOXS beamline is the home of Photon-In, Photon-Out X-ray Spectroscopy at CHEXS. PIPOXS delivers high flux beams in small, focused spot sizes, using a combination of CHESS compact undulator technology, cryogenic silicon optics, and a custom-built double-focusing mirror system. The mission of PIPOXS is to use these beams for studies of catalyst materials inside operating devices. The work of Zeng et al used x-ray absorption spectroscopy measurements of the cobalt K-edge to precisely monitor real-time changes in the valence state of cobalt as the fuel cell voltages were cycled, as well as changes in the Co-N/O and Co-Co bond distances. This was facilitated by integrating a custom electrochemical cell with built-in x-ray windows into the PIPOXS beamline.
How was the work funded?
The Center for High Energy X-ray Sciences (CHEXS), NSF (DMR-1829070)
The Cornell High Energy Synchrotron Source (CHESS), NSF (DMR-1332208)
The Center for Alkaline Based Energy Solutions (CABES), DOE EFRC (DE-SC-0019445)
The Cornell Center for Materials Research (CCMR), NSF MRSEC (DMR-1719875)
Reference:
Nonprecious transition metal nitrides as efficient oxygen reduction electrocatalysts for alkaline fuel cells
Rui Zeng, Yao Yang, Xinran Feng, Huiqi Li, Lauryn M Gibbs, Francis J DiSalvo and Héctor D Abruña
Science Advances 8 5 (2022); https://doi.org/10.1126/sciadv.abj1584