What is the discovery?
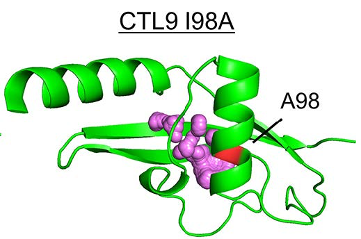
Developing a better understanding of protein folding and unfolding reactions is a significant challenge for structural biology. In a new paper, a team lead by Catherine Royer from Rensselaer Polytechnic Institute reports a high-pressure small-angle x-ray scattering study of the model protein CTL9-I98A in solution, which allows direct observation of the pressure-induced unfolded state. The team was able to study the temperature evolution of the unfolded state and observe that the compactness is not significantly different from proteins which are unfolded by other (chemical) means. This observation runs contrary to expectations and prior theory, and it highlights the utility of direct structural studies in high pressure as a key perturbation for understanding protein unfolded states.
Why is it important?
These experiments demonstrate that at all temperatures, the high pressure unfolded state appears to be slightly less (rather than more) compact than the unfolded state at ambient pressure, suggesting that residual interactions in the unfolded state ensemble are disrupted at high pressure. This is perhaps the first reported study of the temperature dependence of the dimensions of the high pressure unfolded state of a protein. These measurements strongly support the notion that pressure unfolded proteins do not differ significantly from proteins which are unfolded at atmospheric pressure. This suggests that pressure offers a minimally invasive, highly reversible means to populate unfolded states over a range of temperatures, thus allowing fundamental conformational preferences and dynamics to be interrogated.
Why did this research need CHEXS?
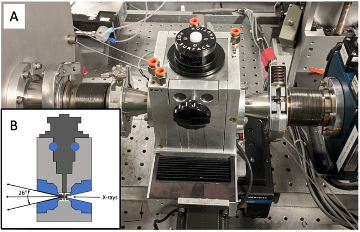
The HPBio research program at CHEXS is dedicated to advancing knowledge in structural biology in the high-pressure regime. The HP-BioSAXS capabilities at beamline 7A1 are ideal for tracking the evolution of biomolecules in solution as a function of pressure and temperature. The combination of CHESS-U undulator beams, high-flux multilayer optics, compound reflective lenses, and a state-of-the-art in-vacuum Eiger4M detector all enable these pioneering experiments to take place.
How was the work funded?
The work was supported by an award from the National Science Foundation MCB 1514575 to and NIH grant GM 078114. This work is based upon research conducted at the Center for High Energy X-ray Sciences (CHEXS), which is supported by the National Science Foundation under award DMR-1829070, and the Macromolecular Diffraction at CHESS (MacCHESS) facility, which is supported by award 1-P30-GM124166-01A1 from the National Institute of General Medical Sciences, National Institutes of Health, and by New York State’s Empire State Development Corporation (NYSTAR).
Reference:
Protein unfolded states populated at high and ambient pressure are similarly compact - Balasubramanian Harish, Richard E. Gillian, Junjie Zou, Jinqiu Wang, Daniel P. Raleigh, and Catherine A. Royer Biophysical Journal (2021); https://doi.org/10.1016/j.bpj.2021.04.031