What is the discovery?
The biological functions of many proteins require transitions between conformational substates. Despite their functional importance, protein conformational substates are often difficult to characterize. In a new paper appearing in Communications Biology, prof. Daniel Keedy's group from the CUNY Advanced Science Research Center used x-ray crystallography at CHESS to investigate the conformation changes of a medically-important biomolecule (the protein tyrosine phosphatase enzyme STEP) when perturbed by pressure and temperature. They produced a first-ever atom-by-atom comparison of the enzyme structure under three conditions: a conventional cryogenic structure (at ~100 K), a physiological-temperature structure (~37 C, or ~310K), and a high-pressure structure (205 MPa). The paper (first-authored by graduate student Liliana Guerrero) reports subtle changes (such as conformational disorder) produced by temperature and pressure. This is the first reported high-pressure structure of a phosphatase. Importantly, the conformational changes induced by pressure and temperature were distinct. While the high-temperature structure shifted the structure more toward an "active-like" conformation (according to torsion angle analysis), the conformation discovered at high pressure had not been seen before. High pressure appears to stabilize a conformation of the active-site loop that is associated with allosteric activation in previous studies, where STEP was crystallized with a small-molecule allosteric activator.
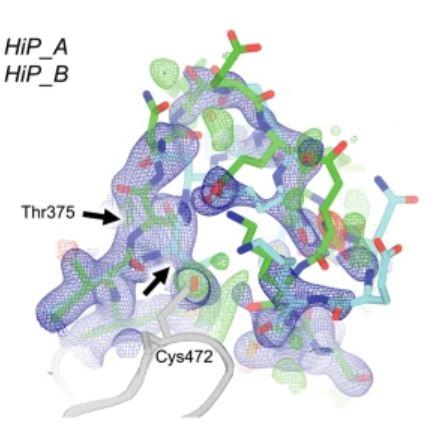
Fig. 1 | Overlay of all three new STEP structures. LoTP = Cryogenic, low pressure;
HiT = High temperature; HiP = High pressure
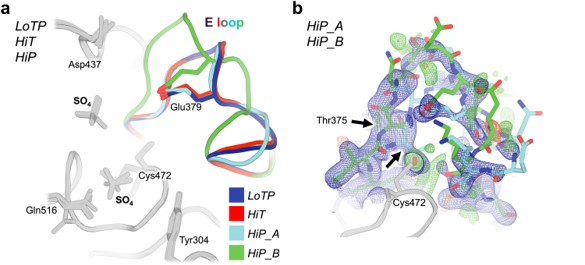
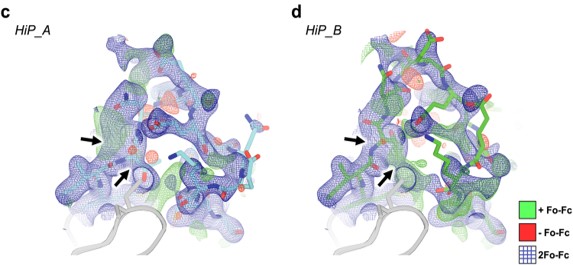
Black arrows indicate Thr375 deviation in all maps.
Why is this important?
STEP is found in the brain and is a therapeutic target for many diseases including Alzheimer's, Fragile X syndrome, and Parkinson's disease. All previously-determined structures of STEP were at conventional cryogenic temperature, however cryo-cooling protein crystals can create artifacts in the structure and limit the accessibility to some confirmations. Determining structures at physiological temperature is a challenge (at ambient temperature, proteins damage more rapidly due to x-ray radiation damage) yet doing so is important to understand proteins in the body. Additionally, perturbative structure determination methods (using variable temperature, pressure, etc.) can reveal conformational states that are low-populated under ambient conditions, and thus hidden from view. Perturbation-based methods that explore non-ambient or even extreme conditions create new opportunities to better understand enzyme structure and function, and lay the fundamental biophysical groundwork needed to discover new potential binding sites for drugs.
Why did this research need CHEXS?
Beamline ID7B at CHESS (FlexX flexible crystallography / XBIO extreme biology) is a dedicated user facility specializing in non-standard protein crystallography experiments, including multi-temperature crystallography, serial crystallography, high pressure crystallography, and crystallography in other extreme environments. CHEXS specifically supports structural biology in extreme conditions, with a dedicated suite of unique capabilities and sample environments. All structures reported in this paper were collected at ID7B beamline. The high-pressure structure was determined using the high-pressure cryo-cooling method, which was developed at Cornell. CHESS is the only facility in North America offering high-pressure cryo-cooling for protein crystals. Bright undulator beams, world-class x-ray detectors, and advanced data analysis pipelines are deployed at ID7B, in cooperation between the NSF and NIH, supporting fundamental biological and biomedical research.
How was the work funded?
The Center for High Energy X-ray Sciences (CHEXS), NSF MPS/BIO/ENG (DMR-1829070)
Macromolecular Diffraction at CHESS (MacCHESS), NIGMS/NIH (1-P30-GM124166-01A1)
New York State’s Empire State Development Corporation (NYSTAR)
NIH R35 GM133769
Reference
Pushed to extremes: distinct effects of high temperature versus pressure on the structure of STEP
Liliana Guerrero, Ali Ebrahim, Blake T Riley, Minyoung Kim, Qingqiu Huang, Aaron D Finke & Daniel A Keedy
Communications Biology 7, 59 (2024). https://doi.org/10.1038/s42003-023-05609-0