What is the new discovery?
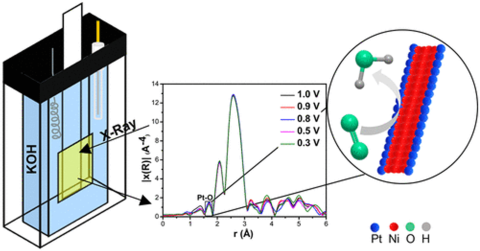
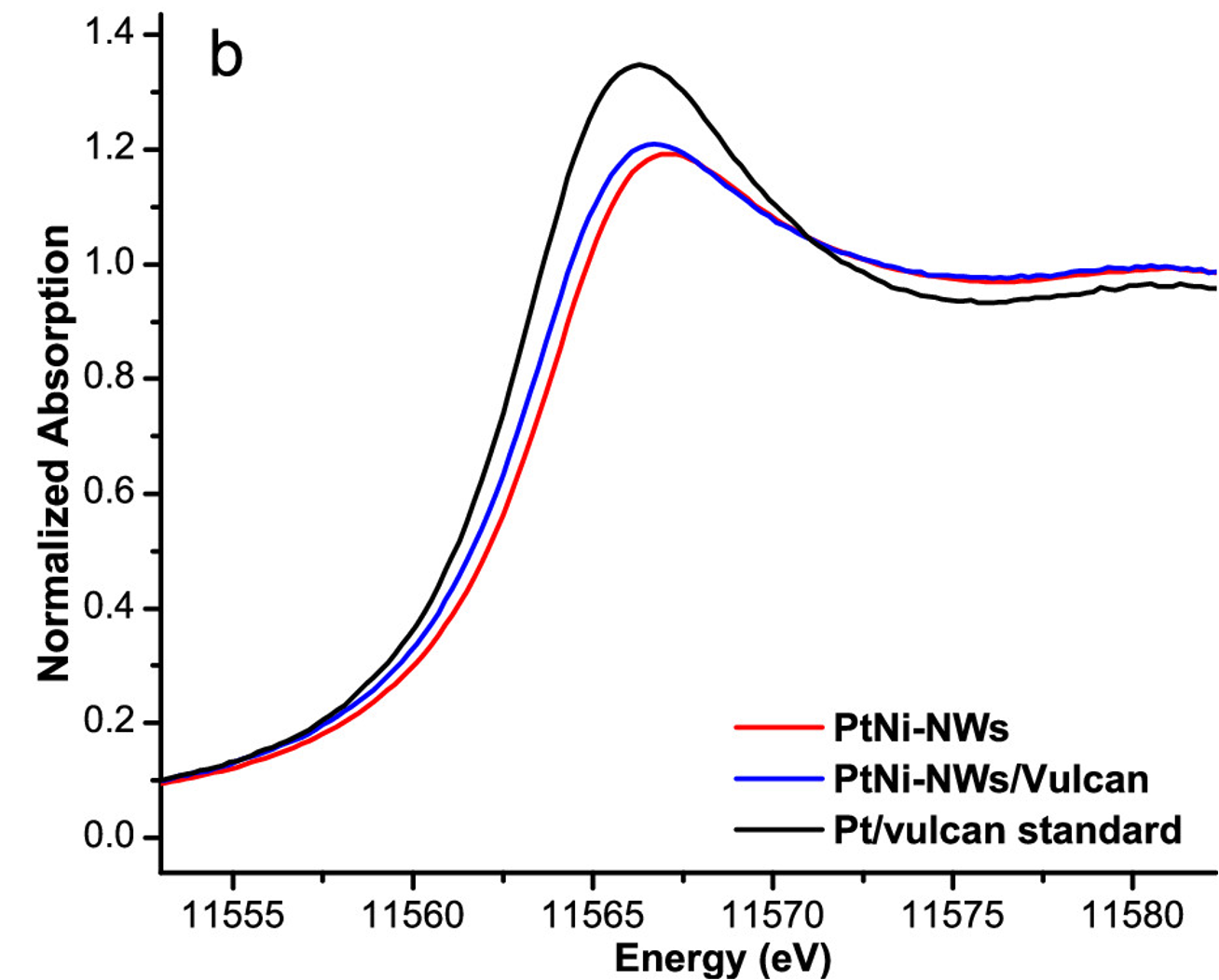
The oxygen reduction reaction (ORR) is an important and often limiting component of hydrogen fuel cell operation. To facilitate this reaction, platinum-based catalysts are often used to increase its rate, though the expense and limited availability of Pt present challenges to its widespread use. In this work, researchers selectively replaced a portion of the nickel atoms of nickel nanowires with platinum to create platinum-nickel nanowires (PtNi-NWs) as high surface area catalysts that reduced the total amount of platinum required. These PtNi-NWs were found to be highly active, and so operando x-ray absorption spectroscopy and extended x-ray absorption fine structure (EXAFS) experiments were conducted at the PIPOXS beamline to assess the electronic and geometric changes occurring in these catalysts during their use. These data enabled the researchers to determine that the Pt formed an alloy with the Ni in the NW and that its interaction with oxygen remained constant regardless of the external potential applied.
Why is it important? What are the Broader Impacts?
Efforts to reduce dependence on fossil fuels require energy generation from alternative sources. Hydrogen fuel cells are one such alternative, though this technology generally requires expensive Pt catalysts in order to function. This work investigates a strategy for reducing the amount of Pt required, while still retaining high catalyst activity. Understanding the atomic-level mechanism of this catalyst serves as an important foundation for the development and commercialization of more efficient fuel cell technology.
Why CHEXS?
The PIPOXS beamline at CHEXS is optimized for the operando study of catalytic systems, making it well-suited for investigating these materials. The flexible nature of the beamline enabled us to accommodate a working electrochemical cell so these catalysts could be studied under their operating conditions, using bright, focused undulator beams at the Pt L3 absorption edge. This work was enabled by the partnership between CHEXS and the Center for Interfacial Electrochemistry of Energy Materials (CiE2M).
How was the work funded?
This work is based upon research conducted at the Center for High Energy X-ray Sciences (CHEXS), which is supported by the National Science Foundation under award DMR-1829070. Additional support was provided by the National Science Foundation NSF-PREM: Center for Interfacial Electrochemistry of Energy Materials (CiE2M) grant number DMR-1827622. The Cornell Center for Materials Research Shared Facilities is supported through the NSF MRSEC grant number DMR-1719875. The 7-BM QAS beamline of the NSLSII is a U.S. Department of Energy (DOE) Office of Science User Facility operated by Brookhaven National Laboratory under contract no. DE-SC0012704.
Reference:
In Situ X-ray Absorption Spectroscopy of PtNi-Nanowire/Vulcan XC-72R under Oxygen Reduction Reaction in Alkaline Media
Joesene Soto-Pérez, Luis E. Betancourt, Pedro Trinidad, Eduardo Larios, Arnulfo Rojas-Pérez, Gerardo Quintana, Kotaro Sasaki, Christopher J. Pollock, Louise M. Debefve, and Carlos R. Cabrera
ACS Omega (2021); https://pubs.acs.org/doi/10.1021/acsomega.1c00792