What is the discovery?
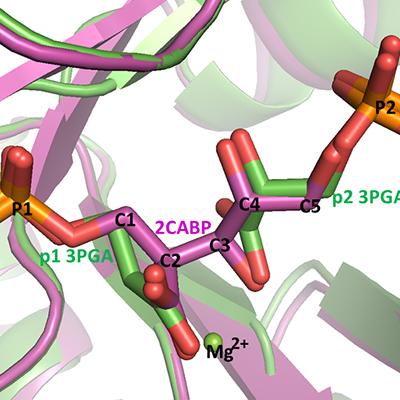
There are four forms of the enzyme Ribulose-bisphosphate carboxylase oxygenase (referred to simply as “Rubisco”) found in nature, and while three of the forms, Types I, II, II, have shown carboxylase/oxygenase activities, Type IV does not and is considered a Rubisco mimic. Recently, MacCHESS researchers Qingqiu Huang and Marian Szebenyi solved the crystal structure of Rubisco protein from Archaeoglobus fulgidus (afRubisco) in complex with two 3-phosphoglycerate (3-PGA) molecules, at a high resolution of 1.7Å. By experimentally determining the enzymatic activity, analyzing the amino acid sequence and comparing the structure with that of a known Type III Rubisco (tkRubisco), Huang deduced that this afRubisco belongs to Type III, whereas it was earlier believed to be a Type IV Rubisco. Because this Rubisco has very low activity, Huang suggests that the enzyme has been trapped in one of its intermediate states, with two bound molecules of the product, 3-PGA, and the enzyme in its closed conformation (Figure 1). The afRubisco/3-PGA complex, like the complex of tkRubisco with the transition state analog 2CABP, mimics an intermediate stage of the carboxylation reaction: after the production of the two 3-PGA products but before the reopening of the active site. This work is helpful to further our understanding of the catalysis mechanism of Rubisco and hence to promote the bioengineered improvement of Rubisco.
Why is it important?
Increasing agricultural productivity is a global development goal. Crop yield is a commonly used metric which depends on three main measures: the efficiency of light energy trapping, the efficiency of light energy conversion into biomass (otherwise known as photosynthesis), and the way carbon is distributed within the plant into what’s eventually harvested. Plants and other organisms use photosynthesis to convert light energy into chemical energy that can later be used to fuel the organisms' activities. This chemical energy is stored in carbohydrate molecules, such as sugars, which are synthesized from carbon dioxide (CO2) and water. In most cases, oxygen is then released as a waste product (Figure 2).
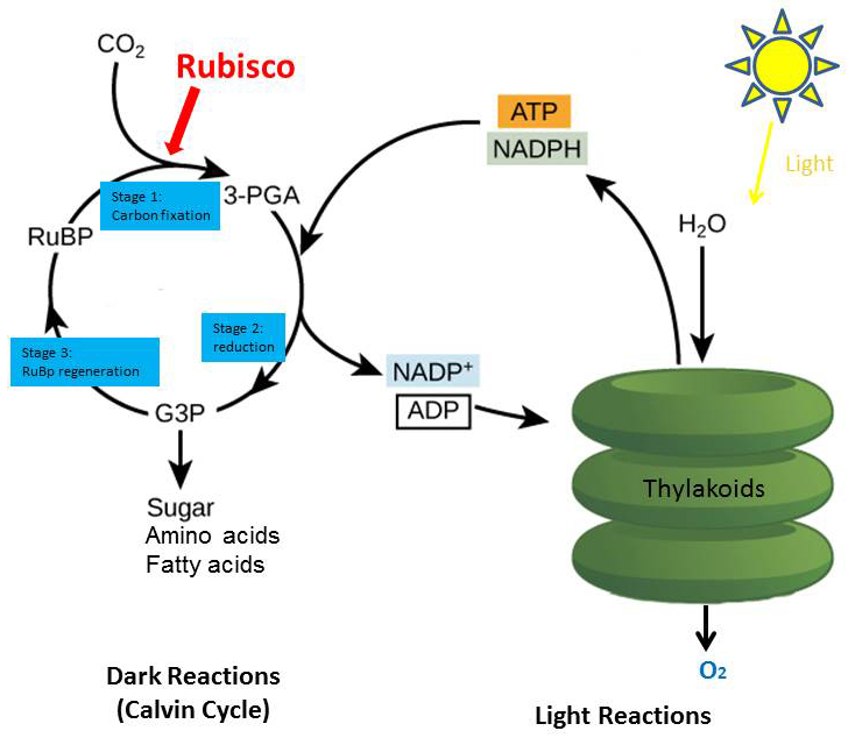
One of the most critical and regulated steps of the photosynthetic process is the carbon dioxide fixation catalyzed by Rubisco. This reaction consists of the incorporation of carbon dioxide to Ribulose-1,5-bisphosphate (RuBP) in a process known as “carboxylation”. Next, Rubisco clips the combined, longer chain into two molecules of 3-PGA. Many processes in the cell require these 3-PGA molecules, so they are typically shuttled away for other pathways, such as making short-term energy storage (in simple sugars), or more long-term stored in the organism as starch. This is the simple process by which atmospheric carbon dioxide is converted by plants and other photosynthetic organisms to energy-rich molecules such as glucose (sugar). In fact, Rubisco makes up somewhere between 30-50% of a plant leaf and is the most abundant single enzyme on Earth!
In spite of this abundance, it exhibits a painfully slow rate of capturing and fixing carbon dioxide to RuBP. Most enzymes can process about a thousand molecules a second, but Rubisco only processes three carbon dioxide per second. To make matters worse, the shape of Rubisco’s carbon dioxide binding site also fits another slightly smaller, but similar shaped molecule competitor, oxygen. Rubisco can fix oxygen to RuBP (oxygenation) to form two molecules, one of 3-PGA and one molecule of 2-phosphoglycerate (2-PG). This process results in a net loss of fixed carbon and reduced efficiency of photosynthesis overall.
Despite its slow rate and low specificity, the importance of Rubisco in photosynthesis (and in improving crop yields) is well recognized. The enzyme has been studied extensively by biochemistry, molecular biology and crystallography for decades. However, only limited progress has been made toward a complete understanding of the details of the catalysis mechanism at the molecular level, mainly due to the complexity of the multiple-step reaction catalyzed by Rubisco. Most of the steps in the reaction mechanism are speculative because of the lack of good models for all stages. Therefore, finding complexes with stable analogs of the intermediate steps is essential for our understanding of the reaction catalyzed by Rubisco. Such analogs have not yet been found for most intermediates.
Why did this research need CHESS?
Station ID7B2 provided the state-of-the-art equipment and excellent support required to obtain the high-quality macromolecular data used in the work. In future, the authors will use the high-pressure cryocooling system at CHESS to investigate the interaction between Rubisco and carbon dioxide and oxygen, which will provide useful information for bioengineering a more efficient Rubisco, i.e. one with both high carboxylation activity and low oxygenation activity.
How was the work funded?
The research was supported by the National Science Foundation award DMR-1829070, the National Institute of General Medical Sciences award 1-P30-GM124166-01A1National Institutes of Health, and by New York State’s Empire State Development Corporation (NYSTAR).
Reference:
Qingqiu Huang and Doletha ME Szebenyi.
Crystal structure of a type III Rubisco in complex with its product 3-phosphoglycerate
Proteins: Structure, Function, and Bioinformatics (2022).1-8; https://doi.org/10.1002/prot.26431